Appendix B: 1,4-Dioxane Identifiers and the Physical and Chemical Properties of 1,4-Dioxane
Table 1. 1,4-Dioxane Identifiers
| Chemical Name | 1,4-dioxane |
| CAS Registry No. | 123-91-1 |
| NIOSH RTECS No. | JG8225000 |
| FDA UNII | J8A3S10O7S |
| DOT ID and Response Guide No. | 1165; Guide 127 |
| USEPA Hazardous Waste ID No. | U108 |
| PubChem CID | 31275 |
| Beilstein Reference No. | 102551 |
| Other Names | Para-Dioxane, p-Dioxane, 1,4-Diethylene dioxide, 1,4-Dioxacyclohexane, Di(ethylene oxide), Diethylene dioxide, Diethylene ether, Diokan, Dioksan (Polish), Diossano-1,4 (Italian), Dioxaan-1,4 (Dutch), Dioxan, Dioxan-1,4 (German), Dioxane, Dioxane-1,4, Dioxanne (French), Dioxyethylene ether, Glycol ethylene ether, NCI-C03689, p-Dioxan (Czech), RCRA waste number U108, Tetrahydro-1,4-dioxin, Tetrahydro-p-dioxin (NIH 2020) |
| Chemical Formula | C4H8O2 |
| Chemical Structure | 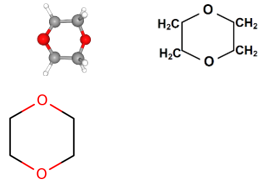 |
| SMILES | O(CCOC1)C1 |
| Major Chemical Class | Heterocyclic organic compound, classified as an ether |
| Physical State, Color, Odor at 15°C | Colorless liquid that can also be a solid below 53°F (11.67°C). Very flammable, volatile liquid with a faint, pleasant, ether-like odor. Vapor harmful. (O’Neil et al. 2001; USCG 1999; ATSDR 2012; ECB 2002; Montgomery 2007) |
| Grades | ACS Reagent (≥99%), anhydrous (99.8%), HPLC (≥99.9%), technical (≥95%) (Sigma Aldrich Co. 2019) |
| Common Impurities | Bis(2-Chloroethyl) ether (<0.1%); Water (<0.1%); 2-Methyl-1, 3-dioxane (<0.1%); 2-Ethyl-1, 3-dioxane (≤0.001%); Hydrogen peroxide (≤0.001%); Lead (≤0.25 ppm); Iron (0.25 ppm) (ECB 2002) |
| Preparation | Commercially prepared by heating diethylene glycol in the presence of sulfuric acid (H2SO4 ) (Surprenant 2012; American Chemical Society 2019) |
| Molecular Weight | 88.106 g/mole (Riddick, Bunger, and Sakano 1986; Mackay et al. 2006; Daubert et al. 1989) |
| Other | Hydrogen bond acceptor count = 2; Covalently bonded unit count = 1; Polar surface area = 18.5Å2. May form explosive peroxides (especially when anhydrous) (NCBI 2020). |
Table 2. Physical and Chemical Properties of 1,4-Dioxane
| Property | Units | Value | Source |
| Acid dissociation constant pKa | No units | –2.92 | (Mohr et al. 2010) |
| Autoignition temperature | °C | 180 | (USCG 1999; Daubert et al. 1989) |
| Azeotropic data for 1,4-dioxane–water mixture | At boiling point = 87.82°C | 82% w dioxane 18% w water | (Horsley 1947) |
| Boiling point | °C | 101.5 | (Lide and Haynes 2009) |
| Critical pressure | MPa | 5.21 (51.4 atm) | (Riddick, Bunger, and Sakano 1986; USCG 1999; Daubert et al. 1989) |
| Critical temperature | °C | 314 | (Daubert et al. 1989; Riddick, Bunger, and Sakano 1986; USCG 1999) |
| Diffusivity in air | (x 10-2 cm2/sec at 25°C) | 8.7 | (USEPA 2001)[MG1] |
| Diffusivity in water | (x 10-5 cm2 /sec at 25°C) value strongly dependent on composition of aqueous phase | 0.32 to 2.38 | (Leaist et al. 2000; Montgomery 2007) |
| Ebullioscopic constant | °C kg/mol | 3.01 | (Lide and Haynes 2009) |
| Electric dipole moment | debyes | 0.4 | (Mohr et al. 2010) |
| Flammability limits in air | Vol % 1 atm, 25°C | 2 to 22% | (Coronado et al. 2012; Daubert et al. 1989; Surprenant 2012) |
| Flash point | °C (closed cup) | 12.2 | (Daubert et al. 1989; USCG 1999) |
| °C (open cup) | 23.3 | (USCG 1999) | |
| Freezing point | °C | 11.8 | (Riddick, Bunger, and Sakano 1986; USCG 1999) |
| Gas phase (atmospheric) OH radical rate constan | (x 1012 cm3 molecule-1-sec-1 at 25°C) | 10.91 +/- 0.5 | (Atkinson 1989) |
| Heat capacity | J mol-1 K-1 at 25 C | 150.65 | (Grolier, Inglese, and Wilhelm 1984) |
| Heat of fusion | kJ/mol | 12.46 | (Riddick, Bunger, and Sakano 1986; Mohr et al. 2010; O’Neil et al. 2001) |
| Heat of vaporization | kJ/mol | 38.60 at 25°C | (Lide and Haynes 2009) |
| 34.16 at 101.5°C | (Lide and Haynes 2009) | ||
| Henry’s law volatility constant | Pa m3/mol at 25°C | 0.486 (4.80 × 10−6 atm m3/mol) | (USEPA and SRC 2019; USEPA 2018[MG2] ; Park et al. 1987; NCBI 2020; ATSDR 2012) |
| Hildebrand solubility parameter | x 104 (J/m3 )1/2 | 2.0163 | (Daubert et al. 1989) |
| Ionization potential | eV | 9.19 +/- 0.01 | (Fraser-Monteiro et al. 1982) |
| Liquid density | g/cm3 at 20°C | 1.0337 | (Lide and Haynes 2009) |
| g/cm3 at 25°C | 1.028 | (Abraham et al. 1971; Hovorka et al. 1936; Reyes et al. 2003; Riddick, Bunger, and Sakano 1986) | |
| Liquid molar volume | cm3/mol | 85.7 | (Daubert et al. 1989) |
| Liquid surface tension | mN/m at 25°C | 32.75 | (Rafati et al. 2009; Jasper 1972; Lide and Haynes 2009) |
| Liquid thermal conductivity | W/m K at 25°C | 0.159 | (Lide and Haynes 2009) |
| Liquid viscosity | mPa s at 25°C | 1.177 | (Lide and Haynes 2009) |
| n-octanol/air partition coefficient, log KOA | No units at 25°C | 3.17 | (Abraham et al. 2001; Mackay et al. 2006; USEPA et al. 2019) |
| n-octanol/water partition coefficient (Log KOW) Odor threshold |
No units Weight/volume (w/v) |
–0.27 at 20°C | (Hansch and Leo 1979; Barone, Rowe, and Quigley 1992) |
| –0.42 at 20°C | (Collander 1951; Chou and Jurs 1979) | ||
| 230 ppm in water | (Amoore and Hautala 1983) | ||
| Volume/volume (v/v) | 24 ppm in air | (ATSDR 2012) | |
| Organic carbon-water partition coefficient (Log KOC) | No units | 0.42 | (USEPA and SRC 2019) |
| 1.23 | (HSDB 2019; Schüürmann, Ebert, and Kühne 2006; Huuskonen 2003) | ||
| 1.46 | (Barone, Rowe, and Quigley 1992; HSDB 2019) | ||
| Refractive index | No units (at 20°C) | 1.4224 | (Lide and Haynes 2009) |
| Relative evaporation rate | Relative to butyl acetate = 1 | 2.42 | (Mohr et al. 2010) |
| Relative to Diethyl Ether = 1 | 7.3 | (NICNAS 1998) | |
| Relative permittivity or dielectric constant | No units at 25 °C | 2.209 | (Papanastasiou, Papoutsis, and Kokkinidis 1987; Maryott and Smith 1951) |
| Standard heat of entropy | J mol-1 K-1 | 270.2 | (Lide and Haynes 2009) |
| Standard heat of formation—liquid | kJ/mol | –355.13 +/- 0.86 | (Byström and Månsson 1982) |
| Trouton constant | J mol-1K-1 | 21.90 | (O’Neil et al. 2001) |
| Ultraviolet light absorption maximum | nm | 180 | (Mohr et al. 2010) |
| Vapor density (relative to air = 1) | No units at 25°C | 3.06 | (Mohr et al. 2010) |
| Vapor pressure | kPa at 20° C | 3.90 (29 mm Hg) | (NIOSH 1994; Crenshaw et al. 1938) |
| kPa at 25°C | 5.08 (38.1 mm Hg) | (Banerjee, Howard, and Lande 1990; Daubert et al. 1989; USEPA et al. 2019) | |
| Water solubility | g/L at 25°C | 1,000 | (USEPA and SRC 2019; Riddick, Bunger, and Sakano 1986) |
| Other solubility data | No units | Soluble in acetone, alcohol, benzene, and ether. Miscible with most organic solvents, including 2-methylpropanol, toluene, cyclohexanone, and cyclopentanone. | (Weast and Astle 1986; Huntress and Mulliken 1941) |
Notes:
Most properties have been obtained from commonly used handbooks, such as the CRC Handbook of Chemistry and Physics (Lide and Haynes 2009), The Merck Index (O’Neil et al. 2001), Physical and Thermodynamic Properties of Pure Chemicals (Daubert et al. 1989), and others. Other properties, such as aqueous solubility, vapor pressure, octanol-water partition coefficient, and Henry’s law constant, have been obtained from scientific journals or other environmental handbooks. Other properties were also obtained from peer-reviewed data sets, such as PubChem (NCBI 2020), the USEPA chemistry dashboard (USEPA 2019a), USEPA Superfund Chemical specific screening data sets (USEPA 2019b), and others.
The emphasis in this table is on experimentally determined values rather than estimated values. Calculated or correlated values are viewed as being less reliable. The most reliable sources of data are the original citations of valuable experimental data in the reviewed scientific literature.
Properties vary depending on many variables. The aim of these tables has been to gather experimental data with a list of citations to interpret them and select a “best” or “most likely” value.
Scientific literature values for 1,4-dioxane’s half-life were not reported. The half-life in the environment depends not only on the intrinsic properties of 1,4-dioxane but also on the nature of the environmental compartments. Factors such as sunlight intensity, hydroxyl radical concentration, and the nature of the microbial community, as well as the temperature, affect 1,4-dioxane’s half-life. Literature values often do not take these factors into consideration, so it is misleading to document a single reliable half-life.