5. Toxicity and Risk Assessment
The USEPA considers 1,4-dioxane to be a likely human carcinogen (USEPA 2013b). The International Agency for Research on Cancer and the U.S. Department of Health and Human Services classify it as possibly carcinogenic to humans (IARC 1999) and reasonably anticipated to be a human carcinogen (DHHS 2014), respectively.
This section discusses human health and ecological risk assessment and presents the toxicological basis of various 1,4-dioxane reference values used for human and ecological risk assessments. It presents different exposure scenarios and site-specific information that investigators may consider when evaluating human and ecological exposures to 1,4-dioxane in environmental media. In addition, uncertainties relating to toxicity assessments and risk characterization are discussed.
5.1 Human Exposure Assessment
The effects of 1,4-dioxane on human health, as with most other chemicals, depends on the magnitude, frequency, and duration of exposure. The limited environmental monitoring data available suggest that the levels of 1,4-dioxane to which the general public might be exposed are significantly lower than those used in studies with experimental animals. Exposure magnitudes and routes from an impacted environmental site will be site-specific.
To determine the general population’s background exposure to 1,4-dioxane, serum was analyzed for 1,4-dioxane in several rounds of testing in the Centers for Disease Control and Prevention (CDC) National Biomonitoring Program of the National Health and Nutrition Examination Survey (NHANES). Blood collected from a geographically diverse population of U.S. adult residents (approximately 2,000 individuals for each sampling period) found no 1,4-dioxane above the methodology’s limit of detection (LOD: 0.5 ng/mL) for 2009–2010, 2011–2012, and 2013–2014 (CDC 2018). The NHANES study did not evaluate potential exposure sources or routes; however, this study suggests that despite the potential for human exposure to 1,4-dioxane from consumer products or impacted public drinking water, persistent and/or continuous exposure to the general population is infrequent, continuously lower than would result in a serum level of 0.5 ng/mL, or nonexistent altogether. Therefore, significant exposures to 1,4-dioxane most likely occur only from contaminated environmental media or in occupational settings. Additional considerations and limitations in the use of serum biomonitoring data for 1,4-dioxane is discussed below in Section 5.1.3.
5.1.1 Human Exposure Pathways and Conceptual Site Models for Risk Assessment
CSMs for risk assessments integrate evaluation of a contaminant’s physical-chemical properties with how possible exposures to the contaminant occurs. Figure 5-1 and Figure 5-2 present examples of pictorial and graphical CSMs used for risk assessments. These types of CSMs can help develop risk assessment strategies to determine which exposure pathways are the greatest concern for each receptor. Developing CSMs for risk assessment is an iterative process that is refined with environmental sampling verification.
Table 5-1 summarizes potential routes of exposure for humans to 1,4-dioxane; however, exposure routes of concern are always site-specific. In general, humans may be exposed to 1,4-dioxane by ingestion, inhalation, and, less significantly, by dermal routes. Industrial workers can have inhalation and dermal exposure to concentrated solutions of 1,4-dioxane at the workplace; however, dermal absorption across intact skin is expected to be minimal [(Mohr et al. 2020); (ATSDR 2012)]. By contrast, for the residential population, the primary exposure routes for 1,4-dioxane would most likely be ingestion of contaminated water from private and public water supplies.
Indoor air exposures may occur from aerosolized emissions from tap water during activities such as showering and bathing (USEPA 2019i). It is reasonable to assume in a residential shower scenario (typical shower temperature is below 42°C/108°F) that exposure will predominantly occur from ingestion of inhaled water vapor and minor absorption in the nasal passages, as 1,4-dioxane will remain in water at temperatures below 88°C/190°F (O’Neil et al. 2001). As such, the inhalation exposure pathway for 1,4-dioxane is considered a minor exposure pathway in most residential scenarios.
Direct contact and incidental ingestion of 1,4-dioxane-impacted soils may need to be evaluated. However, 1,4-dioxane is not expected to adhere to soil particles and/or persist in surface soil as it quickly leaches into the groundwater. See Section 3.2 for additional fate and transport discussion.
Lastly, significant human exposure via consumption of contaminated biota is not likely unless the plant or animal (terrestrial or aquatic) is from an area with 1,4-dioxane concentration levels that would result in sustained exposures. 1,4-Dioxane is not biopersistent or bioaccumulative (see Section 5.4.2). Once in the body, 1,4-dioxane is broken down into metabolites (see Section 5.3), and both 1,4-dioxane and the metabolites are rapidly excreted in the urine; therefore, there is no expectation of significant risks to humans from the consumption of organisms exposed to 1,4-dioxane.
There may be a potential exposure pathway via plants irrigated with contaminated surface water or groundwater (Mohr et al. 2020), although this has not been demonstrated in real-world settings to be of concern for 1,4-dioxane. As discussed in Section 5.3, 1,4-dioxane can be taken up by plant roots but is expected to volatilize from the foliage.
Table 5-1. Examples of potential human exposure routes for 1,4-dioxane
| Medium | Exposure route | Example scenarios |
| Groundwater | Dermal Ingestion Inhalation |
Showering, bathing, hand washing Drinking water, food preparation Indoor showering (aerosolized water vapor) |
| Surface Water | Dermal Ingestion Inhalation |
Recreational activities, showering, bathing Swimming, drinking water, food preparation Indoor showering (aerosolized water vapor) |
| Soil | Dermal Ingestion Inhalation |
Digging, soil handling, gardening, recreational contact Digging, soil handling, gardening, recreational contact Soil particulates and indoor dust |
| Soil Vapor | Inhalation | Not likely except where pure phase is present |
| Indoor Air | Inhalation | Not likely except where pure phase is present |
| Aquatic Biota | Ingestion | Not likely except for eating fish/shellfish caught in surface water with chronically high 1,4-dioxane concentrations |
| Terrestrial Plants | Ingestion | Not likely except for eating plants growing in soil with chronically high 1,4-dioxane concentrations |
| Terrestrial Animals | Ingestion | Not likely except for eating animals from areas with chronically high 1,4-dioxane concentrations |
a) Pictorial Exposure Pathway CSM
A pictorial exposure pathway CSM for risk assessment is a user-friendly representation of how a contaminant flows from a source area to where human and ecological receptors are potentially exposed. Figure 5.1.1a is a pictorial exposure pathway CSM example used in risk assessment illustrating how a 1,4-dioxane release into the environment may migrate. As described in Section 3.1.2, 1,4-dioxane does not adhere to soil or sediment particles and will efficiently leach into the groundwater. In this exposure pathway CSM scenario, 1,4-dioxane exposures to human and animal receptors are primarily from contact with and consumption of contaminated water.
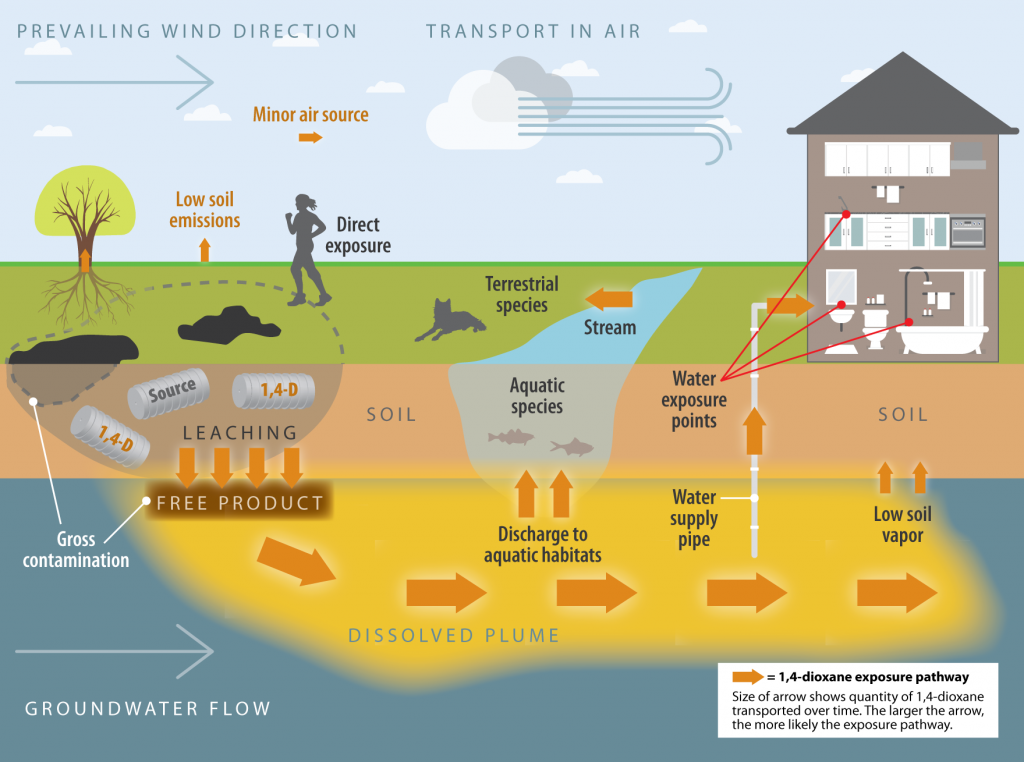
Figure 5-1. Example of a pictorial exposure pathway CSM for risk assessment.
Source: Developed by the ITRC 1,4-Dioxane Team, adapted from https://tphrisk-1.itrcweb.org/5-conceptual-site-models-and-investigative-strategies/
b) Graphical CSM
CSMs for risk assessments may also be represented by graphs of boxes and flow lines depicting exposure pathways from release points to different receptors. For example, Figure 5-2 is a graphical CSM of an exposure scenario from an industry source showing potential exposures to humans and aquatic receptors through aquatic media.
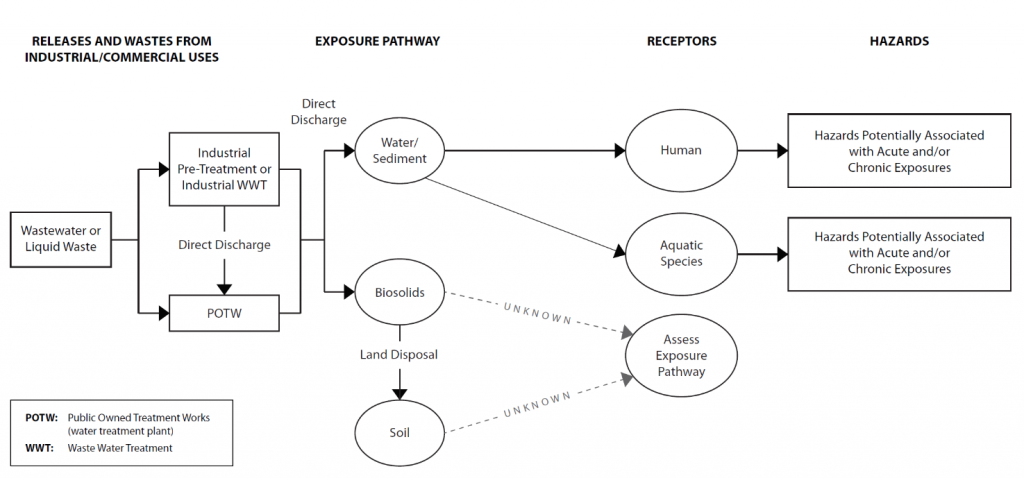
Figure 5-2. Graphical CSM for industrial wastewater releases of 1,4-dioxane and exposure to human and aquatic species.
Source: Developed by the ITRC 1,4-Dioxane Team, adapted from USEPA 2018.
5.1.2 Quantifying Human Exposure
It is not uncommon for USEPA and state governments to have their own exposure assessment equations and exposure factors. Readers should refer to their specific state or USEPA Region for additional exposure assessment guidance. This section provides the reader with references for inhalation, ingestion, and dermal exposure equations.
For general inhalation, ingestion, and dermal exposure assessment formulas, see the USEPA Expo Box webpage (https://www.epa.gov/expobox). See the ITRC website for risk assessment guidance documents that include key topics associated with quantifying exposures (https://www.itrcweb.org/risk-3/#6.%20Exposure%20Assessment.htm#6._Exposure_Assessment%3FTocPath%3D6.%2520Exposure%2520Assessment%7C_____0).
ITRC provides guidance for selecting appropriate exposure factors (https://www.itrcweb.org/risk-3/#6.%20Exposure%20Assessment.htm#6.1__Determining_Appropriate_Exposure_Factors%3FTocPath%3D6.%2520Exposure%2520Assessment%7C6.1%2520%2520Determining%2520Appropriate%2520Exposure%2520Factors%2520%7C_____0).
For recommended values for exposure factors for each exposure route, see USEPA guidance. A summary of the recommended values from the USEPA Exposure Factors Handbook: 2011 Edition and recent updates is provided in spreadsheet format (XLS) for an extensive list of searchable exposure factor tables from the 2011 Handbook (see https://www.epa.gov/expobox/epa-expobox-toolbox-search).
For assessing exposures with user-defined scenarios, the reader is referred to USEPA’s Exposure Factors Interactive Resource for Scenarios Tool (ExpoFIRST). The ExpoFIRST tool is designed for assessors who understand general concepts of exposure assessment. Hyperlinks for the ExpoFIRST tool and supporting documents are below:
- ExpoFIRST Getting Started, Version 2.1 (PDF) (3pp, 131KB, about PDF)
- ExpoFIRST Instructional Notes, Version 2.1 (PDF) (28pp, 482KB, about PDF)
- ExpoFIRST Frequently Asked Questions (FAQs), Version 2.1 (PDF)(24pp, 514KB, about PDF)
- ExpoFIRST Application-Access Database, Version 2.1 (ZIP)(4MB, about ZIP)
- Exposure Factors Interactive Resource for Scenarios Tool (ExpoFIRST), Version 2.0
Ingestion: As discussed above, the most probable 1,4-dioxane exposure scenario for the general population is ingestion of contaminated drinking water from impacted municipal drinking water sources or impacted residential wells (USEPA 2019a). How much 1,4-dioxane exposure occurs when consuming foods prepared with contaminated water has not been well characterized; however, water ingestion rates used in risk assessments typically account for multiple direct and indirect types of exposure from tap water. Ingestion exposure for bottle-fed babies is often an important consideration; therefore, some jurisdictions use a relative source contribution factor to also account for potential dietary ingestion and exposure. We encourage readers to refer to their local state or USEPA Region for ingestion exposure assessment guidance. Parameters such as absorption factors, intake rates, age adjustment factors for early life cancer sensitivity, and others can vary jurisdictionally. Visit the USEPA Expo Box website for a general ingestion exposure assessment formula.
Inhalation: As discussed above, although it is not expected to be a significant exposure pathway, it is possible that the general population may be exposed to 1,4-dioxane through inhalation of ambient air and indoor air in unique scenarios. The assessment of 1,4-dioxane vapor intrusion is unlikely necessary; soil vapor migration from groundwater sources into buildings is unlikely due to 1,4-dioxane’s high water solubility. However, if a building foundation is in direct contact with or flooded with 1,4-dioxane-contaminated groundwater, inhalation exposure may be a concern. For a unique scenario, see the Michigan Department of Environmental Quality information sheet for 1,4-dioxane soil vapor exposure assumptions. For general cancer and noncancer inhalation exposure assessment formulas, visit the USEPA Expo Box webpage.
Dermal: Although dermal exposure from contact with water containing 1,4-dioxane during washing and bathing is a probable scenario, absorption across intact skin is expected to be minimal (Mohr et al. 2020). An example equation for dermal exposure can be found in the ATSDR Water Dermal Contact Dose Equation. For a worker scenario, dermal exposure to concentrated solutions of 1,4-dioxane may pose a greater risk. For a detailed discussion of general dermal exposure assessment and risk, see the USEPA Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment).
5.1.3 Biomarkers of Exposure: 1,4-Dioxane Pharmacokinetics and Metabolism
Metabolic products of xenobiotics are often used as direct measurements of exposure (i.e., biomarkers of exposure). This section describes why 1,4-dioxane and its metabolites are not ideal candidates for use as exposure biomarkers.
1,4-Dioxane is readily absorbed following inhalation or oral exposure. This absorption is generally rapid and complete. The actual absorption mechanism has not been fully identified; however, absorption is thought to occur through passive diffusion (ATSDR 2012). Internal tissue distribution of 1,4-dioxane has not been fully evaluated. Once in the body, 1,4-dioxane is broken down into metabolites, and both 1,4-dioxane and the metabolites are rapidly excreted in the urine (ATSDR 2012). 1,4-Dioxane is extensively metabolized by human cytochrome P450 (CYP450) enzymes (USEPA 2019c). The two primary metabolites found in the urine are 1,4-dioxane-2-one and β-hydroxyethoxyacetic acid (2HEAA). The elimination half-life of 1,4-dioxane in plasma was estimated to be approximately 1 hour in humans and rats, and elimination of HEAA in urine was 2.7 hours in humans and rats (Young et al. 1977). It is not clear whether HEAA or 1,4-dioxane-2-one is the ultimate metabolite (ATSDR 2012); however, recent exposure studies are focusing on HEAA as the primary metabolite (USEPA 2019g).
Ideally, a biomarker of exposure (metabolite) is present only from exposure to the parent compound, and is quantifiable in easily collected body fluid, such as urine or blood. Because of the short biological half-lives, 1,4-dioxane, HEAA, and 1,4-dioxane-2-ol are not ideal candidates for use as exposure biomarkers [(ATSDR 2012); (Mohr et al. 2020)]. The time period for collecting a biological sample to confirm that 1,4-dioxane exposure occurred may be too short for practical use in most clinical settings. The sample collection must occur within days after exposure, before metabolites have been excreted. Additionally, HEAA is not an ideal biomarker of exposure to 1,4-dioxane because it is not specific to 1,4-dioxane metabolism. Exposures to diethylene glycol, a manufacturing precursor of 1,4-dioxane and other organic compounds, will produce HEAA, complicating its use as a specific indicator of 1,4-dioxane exposure. There are also analytical challenges associated with measuring HEAA in serum that may result in either false negative or false positive measurement errors (e.g., HEAA conversion to 1,4-dioxane-2-one under acidic analytical conditions) (ATSDR 2012).
5.2 Human Toxicity
5.2.1 Selection of 1,4-Dioxane Toxicity Values and Their Significant Impact on the Assessment of Human Health Risk
The choice of a 1,4-dioxane toxicity value for use in a human health risk assessment is of critical importance and unique challenges that the risk assessment practitioner must be aware of. Beginning with the USEPA Risk Assessment Guidance for Superfund, Volume I, Human Health Evaluation Manual (Part A), risk assessment guidance recommends selecting toxicity criteria based on the most recent data (USEPA 1989), 7–15). This recommendation has since been implemented and further clarified in numerous USEPA Office of Solid Waste and Emergency Response (OSWER) directives [(USEPA 1993); (USEPA 2003b)] that further establish a hierarchy and process for selecting toxicity criteria. Typically, USEPA Integrated Risk Information System (IRIS) program assessments are considered the top-tier choice (Tier 1) based on their use of standardized methods and because they undergo rigorous interagency and external peer review. However, IRIS toxicity values are not always recent and up to date, nor are they available for all chemicals of potential concern at a site. Tier 2 values are toxicity values developed under the USEPA Provisional Peer-Reviewed Toxicity Value (PPRTV) program by the Superfund Technical Support Center. PPRTVs are developed using the same standardized USEPA risk assessment methods as IRIS values yet are developed in a shorter period of time. PPRTVs do not undergo the same level of peer review as IRIS values; they are typically reviewed internally and then by approximately three external experts via a letter review. Additionally, PPRTVs are often developed using a limited evaluation of chemical-specific information.
Tier 3 toxicity values include any other USEPA (e.g., USEPA Office of Water) and non-USEPA sources of toxicity information (e.g., ATSDR, international values, state assessments, values within a peer-reviewed publication), provided that the information meets certain requirements. The information must be current, peer-reviewed, publicly available, and transparent; it must use standard risk assessment methodology; and the value must have been derived using the best available information[MG7] . For example, ATSDR toxicity values (called “minimal risk levels” [MRLs]) are Tier 3 because they are developed with the same risk assessment methods used by USEPA and they undergo external peer review.
The “tiered” sources of toxicity information are not a de facto prescribed process. Inherent in the selection of the best toxicity value is the flexibility to select the best value at the time that an environmental baseline risk assessment is conducted. Consistent with USEPA directives and guidance documents, priority is given to toxicity information that meets criteria, including transparency of the information and methods, level of external and independent peer review, and use of established methodology consistent with best scientific information and practices used by USEPA. These guiding criteria are presented in the USEPA white paper on selecting Tier 3 toxicity values (USEPA 2013b). This flexibility recognizes that new chemical-specific information may become available, including from international sources, and that risk assessment practices are continually evolving. Online databases exist to facilitate identification of available toxicity values, such as the National Library of Medicine International Toxicity Estimates for Risk (ITER) and the USEPA Chemistry Dashboard (https://comptox.epa.gov/dashboard/. For 1,4-dioxane, it is imperative for the risk assessor to recognize the potential options in toxicity value selection, as the final 1,4-dioxane toxicity value used may have significant quantitative impacts on the assessment of human health risks.
5.2.2 Noncancer Toxicity Values
Noncancer toxicity reference values (reference doses [RfDs] or reference concentrations [RfCs]) of environmental chemicals are estimates of the daily oral or inhalation exposure dose or concentration for a given duration to the human population (including susceptible subgroups) that are likely to be without an appreciable risk of adverse health effects (USEPA 2019e). Noncancer toxicity values, together with human exposure factors, are used to calculate risk-based threshold estimates (e.g., hazard quotient) that are then used to assess potential health hazards resulting from various exposures in environmental media. Estimated risks that exceed agency-defined threshold levels do not have the same implication at all sites and/or situations (ITRC 2015). Therefore, estimates presented in the risk assessment must be properly interpreted and understood in relation to the toxicity values they are based on.
Both oral and inhalation toxicity values are available for 1,4-dixoane. For chronic oral exposures, liver and kidney effects are commonly identified as the most sensitive systemic effect endpoints, and thus serve as the critical effect for most oral toxicity values. For long-term inhalation exposures, atrophy of the olfactory epithelium has been identified as the critical effect from rodent studies. Together, these endpoints account for 1,4-dioxane’s potential effects to the hepatic, urinary, respiratory tract, and nervous systems. Eye and respiratory irritation are the most sensitive effects following shorter-duration inhalation exposure to 1,4-dioxane. USEPA (USEPA 2018e) considered 1,4-dioxane exposures via the soil dermal absorption pathway negligible due to the chemical’s low partitioning (low adsorption) to organic matter (see Table 5-2). However, calculated risk or risk-based screening levels may include default dermal exposure assumptions. Under USEPA, the dermal RfD is generally extrapolated from the oral RfD using a default gastrointestinal absorption efficiency (ABSgi ) value of 100% for organics in the absence of a chemical-specific value. Accordingly, USEPA uses a default ABSgi of 100% to derive the dermal RfD for 1,4-dioxane, which effectively means that the dermal RfD value is equivalent to the oral RfD. Health Canada (EC 2010) uses the same approach to evaluate potential risks resulting from dermal exposure to 1,4-dioxane.
Tables 5-2, Table 5-3, Table 5-4, and Table 5-5 present available noncancer oral and inhalation toxicity values for 1,4-dioxane from various sources, including states and international agencies. Toxicity values vary from among sources for several reasons, including parameters that involve professional judgment, factors based on the database available at the time of the assessment, and risk assessment policy differences between agencies. Additionally, newer understanding of a chemical’s mode or mechanism of action can lead to changes in the interpretation of previously evaluated toxicology data. Some common sources of differences in 1,4-dioxane toxicity values include selection of the critical effect, how the point of departure (POD) was calculated (i.e., use of administered dose or use of benchmark dose [BMD] modeling), and the selection of uncertainty factors.
5.2.2.1 Toxicity Values to Assess Chronic Exposure
Tables 5-2 and 5-3 present the chronic oral and inhalation toxicity values, respectively, from available sources that may be selected by risk assessors for a given chronic (i.e., lifetime) exposure scenario. Section 5.2.1 presents the different toxicity value sources.
Table 5-2. Chronic oral noncancer toxicity values
| Value (mg/kg/day) | Critical effect | Critical study | POD | UF | Adjustments | Source |
| Tier 1: | ||||||
| 0.03 | Liver and kidney toxicity (degenerative effects) | 2-year drinking water study in male and female rats; 60 animals/sex/ dose; and 3 dose levels (Kociba et al. 1974) | NOAEL = 9.6 mg/kg-day in male rats (BMD analysis was not applied due to unreported incidence data) | Total UF = 300 UFA – 10 UFH – 10 UFD – 3 | None | (USEPA 2013b) |
| Tier 3: | ||||||
| 0.0054 | Liver effects (non-neoplastic lesions protective of cancer effects) | 2-year drinking water study in male and female rats; 60 animals/sex/ dose; and 3 dose levels (Kociba et al. 1974) | BMDL5 = 5.4 mg/kg-day for male and female rats combined | Total UF = 1,000 UFA – 10 UFH – 10 UFD – 10 | Relative source contribution of 20% was also applied to derive the proposed maximum acceptable concentration | (HC 2018) (draft) |
| 0.1 | Liver effects (non-neoplastic lesions) | 2-year drinking water study in male and female rats; 60 animals/sex/ dose; and 3 dose levels (Kociba et al. 1974) | NOAEL = 9.6 mg/kg day in male rats (BMD analysis was not used due to lack of incidence data) | Total UF = 100 UFA – 10 UFH – 10 | None | (ATSDR 2012) |
| 0.025 | Liver and kidney lesions (hepatic and renal degeneration and necrosis, regenerative hyperplasia in hepatocytes, and renal tubule epithelial cells) | 2-year drinking water study in rats (Kociba et al. 1974) | NOAEL = 9.6 mg/kg-day | Total UF = 100 UFA – 3 UFH – 10 UFD – 3 | Dose adjustment factor (DAF) = 0.26 | (MNDOH 2013) |
| 0.05 | Hepatocellular necrosis | 2-year drinking water study in rats((Kociba, 1975 #280), which is the laboratory report of (Kociba et al. 1974)) | BMDL10 forthe combined incidence data from male and female rats | Total UF = 100 UFA – 3 UFH – 10 UFD – 3 | None | (Dourson et al. 2014); (ITER 2019) |
Table 5-3. Chronic inhalation noncancer toxicity values
| Value | Critical effect | Critical study | POD | UF | Adjustments | Source |
| Tier 1: | ||||||
| 0.00832 ppm (0.03 mg/m3) | Atrophy of the olfactory epithelium | 2-year (104 weeks) bioassay study in male rats; 50/dose; 3 dose levels (Kasai et al. 2009) | LOAEL = 8.9 ppm, or 32 mg/m3. The BMD results were considered inadequate using non-neoplastic lesions incidence data. PODHEC = 32.2 mg/m3 | Total UF = 1,000 UFA – 3 UFH – 10 UFL – 10 UFD – 3 | Adjusted POD for continuous human exposure (24 hours/day, 7 days/week) over a lifetime and adjustment to a human equivalent concentration (O’Neil et al.) using a DAF of 1 for a systemic acting gas (USEPA 2013b) | (USEPA 2013b) |
| Tier 3: | ||||||
| 0.03 ppm | Atrophy of the olfactory epithelium | 2-year bioassay study in male rats; 50/dose; 3 dose levels (Kasai et al. 2009) | LOAEL = 8.9 ppm, or 32 mg/m3; (inadequate for BMD analysis) LOAELHEC = 8.9286 ppm | Total UF = 300 UFA – 3 UFH – 10 UFL – 10 | Continuous exposure duration adjustment (LOAELADJ = 8.9286) and adjustment to an HEC using DAF = 1 | (ATSDR 2012) |
LOAEL as Lowest Observed Effect Level
5.2.2.2 Toxicity Values to Assess Acute and Shorter-Term Exposure
For short-term exposure to 1,4-dioxane, human health risks can be evaluated using short-term (acute, sub-chronic, or intermediate) toxicity values, presented in Table 5-4 and Table 5-5.
Table 5-4. Short-term and sub-chronic oral noncancer toxicity values
| Value (mg/kg/day) | Critical effect | Critical study | POD | UF | Other adjustment | Source |
| 5.0 (acute oral MRL) | Decreased maternal and fetal body weight and reduced sternum ossification | Developmental study; 17–20 pregnant Sprague-Dawley rats dosed by gavage in water on gestational days 6 to 15; 3 dose levels (Giavini, Vismara, and Broccia 1985) | NOAEL = 516 mg/kg–day | Total UF = 100 UFA – 10 UFH – 10 | None | (ATSDR 2012) |
| 0.5 (intermediate-duration oral MRL) | Liver effects in male rats | 13 weeks of exposure of 1,4-D in drinking water; 10 F344/DuCrj rats/sex/dose; 6 dose levels (Giavini, Vismara, and Broccia 1985) | NOAEL = 52 mg/kgday (data was deemed inadequate for BMD) | Total UF = 100 UFA – 10 UFH – 10 | None | |
| 0.12 (sub-chronic oral RfD) | Increased relative liver and kidney weight (with histological and clinical chemistry changes at higher dose level), hepatocyte swelling, and nuclear enlargement of the nasal respiratory epithelium | 13-week drinking water study in rats (Kano et al. 2008) | NOAEL = 52 mg/kg/day | Total UF = 100 – UFA = 3 UFH = 10 UFD = 3 | Equivalent dose adjustment (HED) using DAF = 0.23 | (MNDOH 2013) |
Table 5-5. Short-term and sub-chronic inhalation, noncancer toxicity values
| Value (ppm) | Type | Basis | Source |
| 2 | 14 days or less acute inhalation MRL | Eye and respiratory effects | ATSDR Acute MRL (ATSDR 2012) |
| 0.2 | 15 to 365 days inhalation exposure MRL | Eye and respiratory effects ((Kasai, 2008 #286) 13-week study) | ATSDR Intermediate MRL (ATSDR 2012) |
| 20 | 8-hr TLV | American Conference of Governmental Industrial Hygienists [(ACGIH 1971); (OSHA 2020)] | |
| 0.28 | 8-hour TWA | California OSHA PEL (CAOSHA 2017) | |
| 1 |
REL for 30-minute ceiling | ((1977; 29CFR 1990); (NIOSH 2019)https://www.cdc.gov/niosh/idlh/123911.html | |
| 3,000 | 8-hour TWA, acute, chronic | Human; respiratory and eye irritation | California Inhalation Acute REL (CAOEHHA 2013a) https://oehha.ca.gov/air/general-info/oehha-acute-8-hour-and-chronic-reference-exposure-level-rel-summary |
| 100 | 8-hour TWA | OSHA PEL (July 1993) (NIOSH 2019) | |
| 500 | Immediately dangerous to life and health (IDLH) | Acute inhalation toxicity | NIOSH IDLH (May 1994) (NIOSH 2019) |
| 17 | Interim AEGL-1 values for 10, 30, and 60 minutes, and 4 and 8 hours | General public could experience mild and increasing odor, taste, and sensory irritation, or certain asymptomatic, nonsensory effects. These effects are not disabling, are transient, and are reversible when exposure is ended. | Acute Exposure Guideline Levels (AEGLs) (emergency airborne threshold levels for the general public) (USEPA 2018a) https://www.epa.gov/aegl/14-dioxane-results-aegl-program (accessed 7/9/2019) |
| 580; 400; 320; 200; 100 | Interim AEGL-2 values for 10, 30, and 60 minutes, and 4 and 8 hours | Public could experience irreversible or other serious, long-lasting adverse health effects, or an impaired ability to escape. | |
| 950; 950; 760; 480; 240 | Interim AEGL-3 values for 10, 30, and 60 minutes, and 4 and 8 hours | Public could experience life-threatening health effects or death. | |
| 1.7 | Level of distinct odor awareness |
AEGL: Acute Exposure Guideline Level; MRL: minimal risk level; NIOSH: National Institute for Occupational Safety and Health; REL: reference exposure level; TLV: threshold limit value; TWA: time-weighted average.
IDLH is based on the acute inhalation toxicity data cited by AIHA, which reported a lethal concentration of 1,000 to 3,000 ppm for 3 hours for guinea pigs, and lethal concentration of 2,085 ppm (8 hours) for mice ([Klimmer 1937, reported by Spector 1956]). (ACGIH 1971) reported that guinea pigs could tolerate 2,000 ppm for several hours without serious symptoms (Yant 1930).
Lower explosive limit (LEL) is based on acute inhalation toxicity data in animals (AIHA 1960; Klimmer 1937).
AEGLs are short-term threshold exposure limits for acutely toxic airborne chemicals used in chemical emergency planning, prevention, and response planning and operations. Interim AEGLs for 1,4-dioxane are available for use while waiting for the National Academies (NRC/NAS) peer review and publication of final AEGLs. Changes to these interim values may occur before the final AEGLs are published.
5.2.3 Cancer Toxicity Values
Typically, cancer risks are the drivers for human health risk assessment for 1,4-dioxane, especially for long-term exposure scenarios. Several different cancer toxicity values (“slope factors”) currently exist for 1,4-dioxane; therefore, risk assessors need to understand the basis of their differences and select the most appropriate toxicity value based on policies, guidance, and site-specific scenarios. Differences in 1,4-dioxane cancer toxicity values can be largely attributed to different interpretations about the mode of action, or how 1,4-dioxane causes cancer in humans (i.e., the shape of the tumor dose-response curve in the low-dose region) and the related dose-response modeling. For agencies that determined that 1,4-dioxane causes cancer via a threshold model (e.g., Health Canada), the resulting regulatory value is significantly higher than those derived based on a linear low-dose cancer model (e.g., USEPA). A general discussion regarding quantitative impacts related to choice of cancer dose-response model and assumptions regarding how a chemical causes cancer (e.g., threshold dose-response model versus linear low-dose model), can be reviewed in more detail in USEPA guidance, including their 2005 Guidelines for Carcinogen Risk Assessment and 2012 Benchmark Dose Technical Guidance.
A summary of each key 1,4-dioxane cancer toxicity factor is below. Importantly, while this discussion focuses on liver tumors and the evidence for a proliferative, regenerative repair MOA, other target organ tumors also have growing supporting evidence for a similar MOA. For example, the rat epithelial mucosa tumors of the nose (the other rodent tumor type of human relevance) possess a robust histopathological data set showing a proliferative regenerative repair MOA basis for this nasal cancer response. The apical outcome (nasal tumors) occurs with dosages in excess of those leading to metabolic saturation of 1,4-dioxane consistent with saturation kinetics as a potential molecular initiating event, as is the case with liver tumors. This information is supported by recent research from Japanese researchers, which provides additional evidence that tumor formation in rodent bioassays has a threshold associated with metabolic saturation (Gi et al. 2018; Furihata et al. 2018; Itoh et al. 2019; Totsuka et al. 2020).
5.2.3.1 USEPA IRIS’ Toxicological Review for 1,4-Dioxane (2010 and 2013)
USEPA evaluated the carcinogenic potential of oral (2010) and inhalation (2013) exposure to 1,4-dioxane and concluded that 1,4-dioxane is “likely to be carcinogenic to humans” by all routes of exposure based on the following evidence:
- Induction of the following tumors in cancer bioassays:([1])
- Liver tumors ((Kano, 2009 #292); (Kasai et al. 2009))
- Peritoneum tumors ((Kano, 2009); (Kasai et al. 2009))
- Mammary gland tumors ((Kano, 2009); (Kasai et al. 2009))
- Kidney tumors (Kasai et al. 2009)
- Zymbal gland (Kasai et al. 2009)
- Subcutaneous fibromas (Kasai et al. 2009)
- Nasal tumors [(Kano et al. 2009); (Kasai et al. 2009); (NCI 1978); (Kociba et al. 1974); (Argus et al. 1973); (Hoch-Ligeti, Argus, and Arcos 1970)]
- Lung tumors (Hoch-Ligeti, Argus, and Arcos 1970)
- No treatment-related tumors in a two-year inhalation study (Torkelson et al. 1974); the finding was attributed to use of only one exposure concentration for the study.
- Inconclusive evidence of a causal link between increased cancer risk and workers’ exposures to 1,4-dioxane; the two available human studies had identified method limitations (small cohort and small number of cancer cases).
In accordance with USEPA’s (USEPA 2005a) Guidelines for Carcinogen Risk Assessment, USEPA IRIS (USEPA 2010) evaluated the available scientific information to determine if a cancer mode of action (MOA)([2]) could be established for 1,4-dioxane. Based on its review of the available evidence, USEPA (2010) concluded that “the available evidence is inadequate to establish a MOA by which 1,4-dioxane induces liver tumors in rats and mice.” Additionally, USEPA (USEPA 2010) concluded that the “MOA by which 1,4-dioxane produces liver, nasal, peritoneal (mesotheliomas), and mammary gland tumors is unknown, and the available data do not support any hypothesized carcinogenic MOA for 1,4-dioxane.” As discussed in Section 5.2.3.2, USEPA’s [(USEPA 2010); (USEPA 2013b)] MOA evaluation for 1,4-dioxane resulted in the agency applying a linear low-dose extrapolation approach for cancer risk assessment.
For oral exposures, USEPA (USEPA 2010) identified three chronic drinking water studies as the most appropriate for quantitative dose-response modeling evaluation [(Kociba et al. 1974); (Kano et al. 2009); (NCI 1978)]. The incidence data for several tumor types—including “either hepatocellular carcinoma or adenoma,” mammary gland adenoma, peritoneal mesothelioma tumors, and nasal squamous cell carcinoma—were modeled using USEPA’s Benchmark Dose Software. Points of departure were converted from administered animal doses to human equivalent doses (HEDs) using a standard body weight scaling factor (based on human body weight of 70 kg) in accordance with USEPA guidance (USEPA 2005a). These results are presented in Table 5.4.1 of USEPA’s (USEPA 2010) Toxicological Review of 1,4-Dioxane. Ultimately, USEPA (USEPA 2010) selected the 95% lower bound on the benchmark dose level (BMDL) associated with a 50% extra risk in “either hepatocellular adenoma or carcinoma” reported in the (Kano et al. 2009) study for female Crj:BDF1 mice as the basis for deriving the oral cancer slope factor (CSF) for 1,4-dioxane. Based on this approach, USEPA (USEPA 2010) calculated the CSF for 1,4-dioxane using “dose-response data for the most sensitive species and gender.” Dividing the benchmark response (50%) by the BMDL50HED (4.95 mg/kg/day) yields an oral CSF of 0.1 (mg/kg/day)-1.
For inhalation exposures, USEPA (USEPA 2013b) identified the chronic inhalation study by (Kasai et al. 2009) as the most appropriate for deriving an inhalation unit risk. The incidence data for several tumor types, including nasal cavity squamous cell carcinoma, hepatocellular adenoma or carcinoma, renal cell carcinoma, peritoneal mesothelioma, mammary gland fibroadenoma, Zymbal gland adenoma, and subcutis fibroma, were modeled individually using the multistage cancer models available in the BMD software. As reported by USEPA (USEPA 2013b):
The best fitting models for each endpoint were used in the BMDS (version 2.2Beta) MS_Combo program to estimate a total tumor BMC and BMCL10 [benchmark concentration associated with a 10% extra risk in total tumors reported]. A Bayesian MCMC analysis was also performed using WinBUGS to calculate the total tumor risk and it yielded similar results (see Appendix G). A summary of the BMDS model predictions for the Kasai et al. (2009) study is shown in Table 5-11. Experimental exposure concentrations were used for BMD modeling and then continuous human equivalent exposures were calculated by adjusting for duration of exposure (Table 5-11) and applying an appropriate DAF [dosimetric adjustment factor] (see Section 5.2.3).
Ultimately, USEPA (USEPA 2013b) selected the 95% lower bound on the benchmark concentration level (BMCL) associated with a 10% extra risk in total tumors reported in the (Kasai et al. 2009) study for male F344 rats as the basis for deriving the inhalation unit risk for 1,4-dioxane. USEPA (USEPA 2013b) reported using this approach because “basing the inhalation unit risk on one tumor site may underestimate the carcinogenic potential of 1,4-dioxane.” Dividing the benchmark response (10%) by the BMCL10 (19.5 mg/m3 or 19,500 μg/m3) yields an inhalation unit risk (IUR) of 5 x 10-6 (μg/m3)-1.
5.2.3.2 USEPA OCSPP Draft Risk Evaluation for 1,4-Dioxane
In response to the LCSA’s requirements, USEPA (USEPA 2019e) reevaluated the carcinogenic potential of oral and inhalation exposure to 1,4-dioxane. As of the writing of this section, the USEPA assessment is draft; therefore, the content and conclusions may change. Similar to USEPA’s IRIS Assessment [(USEPA 2010); (USEPA 2013b)], USEPA (USEPA 2019e) concluded that 1,4-dioxane is “likely to be carcinogenic to humans” based on “animal evidence of carcinogenicity at multiple sites, in multiple species, and multiple routes.” Regarding the cancer MOA evaluation, the draft USEPA document (USEPA 2019e) states:
The relationship between cell proliferation, hyperplasia, and 1,4-dioxane mediated tumor formation has not been established. Though several publications (Dourson et al., 2017; Dourson et al., 2014; McConnell, 2013) do provide evidence of cytoplasmic vacuolar degeneration and hepatocellular necrosis in rat and non-neoplastic lesions, the animal data does not support a dose-response relationship between cell proliferation, hyperplasia, and liver tumors in rat and mouse studies. Kociba et al. (1974) reported hepatic degeneration and regenerative hyperplasia at or below dose levels that produced liver tumors, but incidence for these effects was not reported. Therefore, a dose-response relationship could not be evaluated, and the events cell proliferation and hyperplasia are not supported by available data. Finally, the doses in hepatotoxicity studies where cytotoxicity and cell proliferation were observed were greater than cancer bioassay dose levels. Integrating data across studies, dose-response relationships between cytotoxicity and tumor formation are not well established in the rat and mouse data and are inconsistent among bioassays and across exposure duration.
EPA determined that evidence is not sufficient to support a MOA of cytotoxicity followed by sustained cell proliferation as a required precursor to tumor formation related to the metabolic saturation and accumulation of the parent compound, 1,4-dioxane (Dourson et al., 2017; Kociba et al., 1975). In addition, while genotoxicity is evident from high doses with in vitro and in vivo studies the occurrence at high doses and potential confounding with cytoxicity does not support a mutagenic mode of action hypothesis at low doses in vivo. Other than liver tumors, no plausible MOA has been hypothesized for the other tumor types associated with exposure to 1,4-dioxane.
In the absence of an established cancer MOA, USEPA (USEPA 2005a) recommends using a linear low-dose extrapolation procedure for cancer risk assessment.
For oral exposures, USEPA (USEPSA 2019e) identified three chronic drinking water studies as the most appropriate for quantitative dose-response modeling evaluation [(Kociba et al. 1974); (Kano et al. 2009); (NCI 1978)]. The incidence data for several tumor types were evaluated using the multistage cancer models available in USEPA’s BMD software and included the following tumor types:
- Rats: nasal squamous cell carcinoma, peritoneal mesothelioma, hepatocellular adenoma or carcinoma, subcutis fibroma, and mammary gland adenoma.
- Mice: hepatocellular adenoma or carcinoma.
Additionally, total tumor incidences (including and excluding liver tumors) in male or female rats from (Kano et al. 2009) were evaluated using the MS_Combo model to calculate benchmark doses associated with a specific composite risk for combined tumor types. Note that USEPA (USEPA 2019e) did not model the female mouse hepatocellular carcinoma data from (Kano et al. 2009) “due to the difficulties that were previously noted” by USEPA (USEPA 2013b). USEPA (USEPA 2019e) also states:
Specifically, this endpoint exhibited a low control group incidence, and a high (70% incidence) response rate at the lowest dose followed by a plateau. While the [USEPA (2013)] IRIS assessment did perform BMD modeling on these data, it was necessary to increase the BMR, omit the highest dose group, and apply a non-multistage model.
Points of departure were converted from administered animal doses to HEDs using a standard body weight scaling factor (based on human body weight of 80 kg) and in accordance with USEPA guidance (USEPA 2005a). These results are presented in Table 4-12 of USEPA’s draft risk evaluation (USEPA 2019e). Ultimately, USEPA (USEPA 2019e) selected the BMDL associated with 10% extra risk in total tumors (including liver) reported by (Kano et al. 2009) for male F344/DuCrj rats as the basis for deriving the oral CSF for 1,4-dioxane. Dividing the benchmark response (10%) by the BMDL10HED yields a draft oral CSF of 0.021 (mg/kg/d)-1, which is less than an order of magnitude (i.e., about 5 times) less potent than the oral CSF of 0.1 (mg/kg/d)-1 reported by USEPA (USEPA 2010).
For inhalation exposures, the USEPA draft document (USEPA 2019e) identified the chronic study by (Kasai et al. 2009) as the most appropriate for deriving an IUR([3]). The incidence data for the same tumor types evaluated in the USEPA IRIS (USEPA 2013b) assessment were again evaluated using BMD software, and included nasal cavity squamous cell carcinoma, Zymbal gland adenoma, hepatocellular adenoma or carcinoma, renal cell carcinoma, peritoneal mesothelioma, mammary gland fibroadenoma, and subcutis fibroma. These tumors were modeled individually using the multistage model. The best fitting models for each tumor type then underwent an evaluation using the MS_Combo model to calculate benchmark concentrations associated with a specific composite risk for combined tumor types. The MS_Combo modeling evaluation included five modeling runs on the following tumor groups (USEPA 2019e):
- All portal-of-entry tumors
- All systemic tumors
- Systemic tumors minus the liver tumors
- All portal-of-entry and systemic tumors
- All portal-of-entry tumors and systemic tumors minus the liver tumors
The BMCL values associated with a 10% extra risk from the fourth and fifth MS_Combo model runs were 31.3 ppm and 35.9 ppm, respectively. These values are basically indistinguishable from the BMCL10 developed by USEPA (USEPA 2013b) of 30.3 ppm. As a result, the draft inhalation unit risk value determined using the benchmark dose modeling results reported by USEPA (USEPA 2019e) and associated with continuous exposure is indistinguishable from USEPA’s (USEPA 2013b) current IUR of 5 x 10-6 (μg/m3)-1.
5.2.3.3 Health Canada’s Draft 1,4-Dioxane in Drinking Water Guideline Technical Document
Health Canada (HC 2018) noted that 1,4-dioxane has been classified as a carcinogen by the following national and international bodies:
- The International Agency for Research on Cancer: “Possibly carcinogenic to humans” (group 2B) “based on sufficient evidence in experimental animals and inadequate evidence in humans.”
- The National Toxicology Program: “Reasonably anticipated to be a human carcinogen” based on “sufficient evidence in animals and inadequate evidence in humans.”
- USEPA (USEPA 2013b): “Likely to be carcinogenic to humans” based on “sufficient evidence in animals (including hepatic tumors in multiple species and strains, as well as peritoneal mesotheliomas, mammary gland, and nasal tumors) and inadequate evidence of carcinogenicity in humans.”
Because liver toxicity was deemed the most sensitive endpoint of cancer, Health Canada (HC 2018) evaluated two MOA hypotheses for liver cancer: one involving genotoxicity-induced carcinogenicity, and a second involving regenerative proliferation-induced carcinogenicity. With regard to the genotoxicity-induced carcinogenicity hypothesis, Health Canada (HC 2018) evaluated the available information for this proposed MOA considering (Meek et al. 2014) modified Bradford Hill criteria for dose-response, temporal concordance, consistency and specificity, and biological plausibility. Based on its review of information supporting the genotoxicity-induced carcinogenicity hypothesis, Health Canada (HC 2018) concluded:
The genotoxic mode of action was not able to satisfy the conditions of dose concordance, consistency and specificity, and biological plausibility of the modified Bradford Hill criteria for a plausible MOA (Meek et al., 2014). This analysis indicates that the pattern of genotoxicity is inconsistent with a MOA where genotoxicity is an early and influential key event in the carcinogenic MOA. Similar conclusions were reached by the governments of Canada (Environment Canada and Health Canada, 2010) and Australia (NICNAS, 1998), by the European Union (European Commission 2002), and by the USEPA (2013).
Similarly, Health Canada (HC 2018) applied (Meek et al. 2014) modified Bradford Hill criteria to the key events involved in the regenerative proliferation-induced carcinogenicity hypothesis. The key events involved in this MOA are:
- Key Event 1: Accumulation of parent compound due to metabolic saturation.
- Key Event 2: Liver cell hypertrophy and necrosis.
- Key Event 3: DNA synthesis.
- Key Event 4: Regenerative cell proliferation.
- Key Event 5: Tumor promotion.
For the regenerative proliferation-induced carcinogenicity MOA, Health Canada (HC 2018) concluded:
Dose and temporal concordance are evident upon consideration of multiple studies across different durations. Increased 1,4-dioxane doses were associated with increased tumour incidence in mice and rats, and the key events are observed at doses below or similar to those associated with cancer. The sequence of key events is logical, and the key events and adverse outcomes occur in an expected order. More specifically, histopathological changes indicative of hypertrophy and necrosis are observed following short-term studies and are further observed in chronic bioassays preceding the development of tumours. These key events have been observed in repeated chronic experiments in different laboratories (NCI, 1978; Kano et al., 2009) as evidence for consistency. … Support for the proposed MOA is found by analogy to other solvents that cause liver tumours in both rats and mice. Moreover, all key events in the rodent MOA are concordant and plausible in humans, although limited data are available to provide support.
Health Canada concluded that insufficient data exist to assess potential MOAs for other tumor types; however, it also determined that those tumor types are not relevant to human health nor are they as sensitive as the liver effects. Therefore, based on results from its MOA evaluation, Health Canada (HC 2018) elected to use a nonlinear (threshold) risk assessment approach to evaluate cancer risks associated with drinking water exposure to 1,4-dioxane (see the reference dose presented above in Table 5-2, described further below).
Incidence data for hepatocellular necrosis (Key Event 2) in rats chronically exposed to 1,4-dioxane in the drinking water (Kociba et al. 1974) were selected for calculation of a tolerable daily intake (TDI) because liver toxicity “has been identified as the most sensitive endpoint of concern” (HC 2018). Additionally, Health Canada (HC 2018) considers this approach to be protective of other tumor types. The incidence data for hepatocellular necrosis were evaluated using benchmark dose modeling separately for (1) male or (2) female rats and for (3) male and females combined (Health Canada 2018) and at different benchmark response levels (5% and 10% extra risk). Ultimately, Health Canada (HC 2018) selected the BMDL associated with an extra risk of 5% for hepatocellular necrosis in the combined male and female rat data set as the basis for deriving the TDI (BMDL5 = 5.4 mg/kg/d for males and females combined). Health Canada (HC 2018) then calculated the TDI of 0.0054 mg/kg/d by dividing the BMDL5 by a composite uncertainty factor of 1,000:
- 10x for extrapolating from laboratory animals to humans.
- 10x to account for potentially sensitive subpopulations.
- 10x for deficiencies in the toxicology database (e.g., related to reproductive and developmental toxicity).
Health Canada (HC 2018) did not evaluate the inhalation toxicity potential for 1,4-dioxane, but rather performed on a multiroute exposure evaluation to determine if the relative contribution of this pathway during bathing and showering was significant.([4]) Based on this analysis, Health Canada concluded that exposure to 1,4-dioxane from drinking water via inhalation was not significant and that no further consideration of this exposure pathway was needed.
5.2.3.4 Uncertainty in the 1,4-Dioxane Cancer MOA
The uncertainty in 1,4-dioxane’s cancer MOA has resulted in differences in how the cancer risks associated with exposure to 1,4-dioxane are evaluated and in ongoing scientific debate. As discussed previously, USEPA’s IRIS (USEPA 2013b) and OCSPP (USEPA 2019e) assessments concluded that the available information on 1,4-dioxane’s cancer MOA does not support either a regenerative proliferation MOA or a mutagenic cancer MOA. Specifically, USEPA (USEPA 2013b) states:
In the case of 1,4-dioxane, there is insufficient biological support to identify key events and to have reasonable confidence in the sequence of events and how they relate to the development of tumors following exposure to 1,4-dioxane; thus, the data are not strong enough to ascertain the mode of action applying the Agency’s mode of action framework.
In these situations, USEPA relies on recommendations provided in Guidelines for Carcinogenic Risk Assessment (USEPA 2005a) and defaults to a linear low-dose extrapolation model for evaluating potential cancer risks.
In contrast, Health Canada’s (HC 2018) draft review included a direct comparison of the weights of evidence for a mutagenic cancer MOA and a regenerative proliferation cancer MOA based on modified Bradford Hill criteria (Meek et al. 2014) and led the agency to select the cancer MOA with stronger scientific support as the basis for cancer risk evaluations. Using this approach, Health Canada (HC 2018) concluded that while the genotoxicity cancer MOA “does not meet the modified Bradford hill criteria,” the regenerative proliferation cancer MOA “meets many of these criteria (specifically dose and temporal concordance, consistency, analogy, and biological concordance).” This conclusion led Health Canada (HC 2018) to adopt a threshold model (rather than a linear low-dose extrapolation model) for evaluating cancer risks associated with 1,4-dioxane that is directly linked to the saturation of 1,4-dioxane’s metabolic pathways. Similar approaches have been used by Australia (NICNAS 1998) and the European Union (European Commission 2002).
A couple of important points concerning the nonhepatic livers noted in rats (nasal, kidney, mammary, subcutis fibroma, and peritoneal mesotheliomas) are briefly mentioned here. As previously described, the rat nasal epithelial tumors show a constellation of noncancer toxicity responses that support a threshold key event/MOA explanation as well as a dose response arising from a high-dose outcome following saturation of 1,4-dioxane metabolism. These non-liver tumor types occur at dosages slightly below or slightly greater than doses required to cause liver tumors; the dosages causing tumors generally exceed 1,4-dioxane’s metabolic saturation. For example, the benchmark dose estimates for these tumors, from which a linear extrapolation or uncertainty factors would be applied, are roughly in the same range. USEPA’s BMDL modeling of the (Kano et al. 2009) drinking water study reported the following BMDL10 values for nasal, liver, peritoneal mesothelioma, and subcutis fibroma in male rats: 242, 28.3, 35.4, and 85 mg/kg/day. The BMDL10 modeling of the combined Kano et al. 2009 male rat tumors was 17.8 mg/kg/day. It is notable that Zymbal gland tumors occur in a unique gland found in rodents that humans do not possess. Mammary and kidney cancer are inconsistently elevated and only slightly so across the rat cancer bioassays. Both the peritoneal mesothelioma and subcutis fibroma tumors are relatively common in rats and are likely to be prone to chemically induced tumor promotion versus being relatively rare in humans (see Boorman’s Pathology 2018; Zwicker et al. 1992; Blackshear et al. 2014). Therefore, growing evidence suggests that of the rodent tumors relevant to human health, the same sequelae t are following 1,4-dioxane exposure levels that surpass the metabolic threshold, suggesting a similar MOA.
5.3 Ecological Exposure and Toxicity
5.3.1 Ecological Exposure Assessment
As discussed in Section 3, 1,4-dioxane can occur in air, water, and land (USEPA 2018e). Due to its high solubility, low octanol-water partition coefficient, and low vapor pressure, 1,4-dioxane is expected to partition mainly into aqueous media when released to the environment. There is no indication of bioaccumulation or bioconcentration (ATSDR 2012), but plants do uptake 1,4-dioxane, subsequently releasing the compound from foliage via volatilization. Although there is no accumulation, terrestrial plants may be a sink for the compound during the travel time from root to leaf (Aitchison et al. 2000). 1,4-Dioxane is resistant to biodegradation in the environment and volatilizes from water very slowly (USEPA 2018e). Given 1,4-dioxane’s general fate and transport characteristics (see Section 3), media other than aquatic media (surface water, sediment, and groundwater at discharge zones) are of little ecological concern. Therefore, aquatic receptors are the primary concern for 1,4-dioxane’s ecological risk.
5.3.2 Ecotoxicity Assessment
Consistent with the most likely presence of 1,4-dioxane in aqueous media, ecotoxicity to aquatic receptors is discussed first, followed by terrestrial and other biota. Table 5-6 summarizes toxicity screening levels.
Table 5-6. Potential ecological screening levels for 1,4-dioxane
| Medium | Concentration | Type/media | Reference | Comments |
| Surface Water (fresh water) | 15 mg/L | Chronic COC | (USEPA 2018e) | Based on lowest fish MATC of >145 mg/L and an AF = 10 |
| 57.5 mg/L | PNEC-water | (ECB 2002) | Based on Microcystis blue-green algae test with and AF = 10 | |
| 10 mg/L | PNEC-water | (ECHA 2014) | AF = 10 | |
| 201 mg/L | ChV-algae | (USEPA 2019e) | Calculated using EcoSAR | |
| Sediment | 43.3 mg/kg (ww) | PNEC-sediment | (ECB 2002) | Calculated based on Equilibrium partitioning from water |
| 37 mg/kg (dw) | PNEC-sediment | (ECHA 2014) | Calculated based on PNEC-water and Equilibrium Partitioning between water and sediment | |
| Soil | 14 mg/kg | PNEC-soil | (ECB 2002) | Calculated based on PNEC-water and Equilibrium Partitioning between water and soil |
AF: Assessment factor, a measure of uncertainty applied to the critical value based on the confidence in the data set; ChV: chronic value, calculated via EcoSAR; COC: concentration of concern, calculated as the lowest applicable value in test data with appropriate UF applied; dw: dry weight; EcoSAR: Ecological Structure Activity Relationship;
MATC: maximum acceptable threshold concentration, defined as the geometric mean of the no effect concentration and the lowest effect concentration; PNEC: probable no effect concentration, calculated per ECHA procedures from available test data and application of appropriate uncertainty factors; ww: wet weight.
5.3.2.1 Aquatic Receptors
Acute toxicity
In general, fish are the most sensitive aquatic receptors, with acute lethal concentrations (those impacting 50% of test subjects, LC50) starting at 100 mg/L (96-hour LC50) for the fathead minnow (Pimephales promelas) (USEPA 2018e). The least sensitive reported fish species is the inland silverside (Menidia beryllina), with a 96-hour LC50 of 67,000 mg/L. Two species of water flea, Daphnia magna and Ceridodaphnia dubia, were reported to have 48-hour effect concentrations impacting 50% of test subjects (EC50s) in excess of 1,000 mg/L and 299 mg/L, respectively. Three species of aquatic plants were tested in two separate studies. In the first study, green alga (Pseudokirchnerella subcapitata) did not experience adverse impacts on growth at concentrations as high as 1,000 mg/L (EC50) after 72 hours of static exposure. A no observed effect concentration (NOEC) of 580 mg/L was established for the biomass endpoint. In the second study, cyanobacterium (Microcystis aeruginosa) and green alga (Scenedesmus quadricauda) exposed to 1,4-dioxane at concentrations ranging from 575 to 5,600 mg/L (EC50s) exhibited cell inhibition after 192-hour exposures.
Chronic toxicity
In chronic toxicity tests, fish were also more sensitive than other aquatic receptors. Japanese medaka (Oryzias latipes) were exposed to 565–6,933 mg/L of 1,4-dioxane for 28 days under flow-through conditions. An NOEC for growth and survival was reported as the lowest concentration tested (i.e., 565 mg/L). A chronic toxicity bioassay on hatching, larval development, and larval survival of embryonic fathead minnow exposed to a battery of 1,4-dioxane concentrations ranging from 27.6 to 45 mg/L for 32 days reported an NOEC of >103 mg/L (larval survival). Using these data, a maximum acceptable toxicant concentration (MATC) of 145 mg/L was calculated. A chronic toxicity study on Daphnia magna via a 21-day test revealed an NOEC (reproduction, survival, and growth) of 1,000 mg/L at the highest exposure concentration tested.
Toxicity results for benthic biota were not available. However, pore water into which 1,4-dioxane is likely to partition should be considered in terms of ecotoxicological conceptual exposure models for contaminated sites. It is likely that benthic species would have similar sensitivity to 1,4-dioxane as aquatic invertebrates for which toxicological data are available.
Risk to sediment organisms from 1,4-dioxane exposure is expected to be low. The results for the various aquatic species suggest that even at the most sensitive range for ecotoxicity reported, acute effect levels ≥100 mg/L and chronic effect levels ≥1 mg/L are considered essentially nontoxic according to the Globally Harmonized System’s (UN 2003) hazard classification scheme. Moreover, USEPA’s (USEPA 2018e) risk evaluation in the problem formulation document indicates that the current aquatic pathways are not of nationwide concern for 1,4-dioxane since exposures are below the acute and chronic concentrations of concern of 60 mg/L and 15 mg/L, respectively. For comparison, national-scale monitoring data from USEPA’s Storage and Retrieval (STORET) and National Water Information System (NWIS) show 1,4-dioxane has a detection rate of approximately 6% in surface waters in the United States, with concentrations ranging from 0.0006 to 0.1 mg/L (USEPA 2018e). This is two orders of magnitude below the concentrations of concern.
5.3.2.2 Terrestrial Receptors
Toxicity data for terrestrial species is limited. However, ecotoxicity for mammalian wildlife can be approximated by the existing data from human toxicity testing described in detail in Section 5.2. In brief, 1,4-dioxane is expected to impact wildlife through ingestion, inhalation, and dermal pathways. Eye and respiratory tract irritation may occur after exposure to low levels and for short periods of time. Exposure to very high levels may cause kidney and liver effects and possibly mortality at high levels (human LC100 is approximately 470 ppm). Other wildlife, such as birds, semiterrestrial amphibians, and reptiles, are expected to experience similar effects. Since 1,4-dioxane does not bioaccumulate or bioconcentrate, trophic-level secondary poisoning is not expected.
Terrestrial plant data suggest low sensitivity to 1,4-dioxane. Common lettuce (Lactuca sativa) exposed to 1,4-dioxane in a germination/root elongation toxicity test for 3 days resulted in an EC50 of 1,450 mg/L. The June 20, 2019, European Chemicals Agency (ECHA) dossier for terrestrial plants reports a short-term LC50 of 2,175 mg/kg dry weight soil (https://echa.europa.eu/registration-dossier/-/registered-dossier/15842/6/4/4).
In general, environmental monitoring data suggest that ambient levels of 1,4-dioxane are lower than those associated with adverse effects in experimental animals, including threatened and endangered species, whose status requires added protection to ensure individual survival.
5.4 Risk Characterization and Uncertainty
The final step in the risk assessment process is “risk characterization.” Exactly what this includes is site- and situation-specific and should be fit for purpose. Risk characterization has been described as the bridge between risk assessment and risk management because it provides a basis for the risk management decisions considering the uncertainties inherent in the risk evaluation and the results of the risk assessment steps [(ITRC 2015), Section 7)]. USEPA (USEPA 1995a) notes the following:
The risk characterization integrates information from the toxicity assessment and exposure assessment and synthesizes an overall conclusion about risk that is complete, informative and useful for decision makers.
The challenge of risk characterization is describing uncertainties and limitations in the data gathered for the risk assessment and clearly communicating the key findings and the context of those findings. For more information regarding risk characterization, refer to USEPA’s Human Health Risk Assessment web page (USEPA 2020b) and select Step 4, Risk Characterization.
This section will discuss considerations for the risk characterization and uncertainty analysis for both human health and ecological risk assessments. For general guidance on methods and approaches to risk characterization for ecological risk assessment, see the references noted in Section 5.4.2.
5.4.1Human Health Risk Characterization
For a 1,4-dioxane human health risk assessment, the key areas of uncertainty include the selection of appropriate toxicity values and adequately quantifying exposures.
As presented in Section 5.2, a toxicity value (e.g., an oral RfD or an oral CSF) is a numerical expression of the dose and effect response for a chemical. For 1,4-dioxane, several toxicity values are available for use, following established guidance (ITRC 2015). Section 5.2 discusses the hierarchy of available toxicity values for 1,4-dioxane. The selected toxicity value for a given risk assessment must be consistent with established guidance and policies, must be well justified, and must explain the uncertainties and limitations. It is important to provide insight into the degree of uncertainty and potential bias inherent in the specific toxicity values used in the risk assessment. Areas of uncertainty in toxicity values generally include extrapolation from controlled high-dose animal studies to the human general population (including potentially sensitive subgroups) and generally lower exposure levels, determining if rodent endpoints are relevant to humans (e.g., tumors in the Zymbal’s gland of rodents), route-to-route extrapolation if toxicity values are lacking for certain exposure pathways, extrapolation from shorter-duration exposure studies to chronic or lifetime risk, and data gaps in the underlying toxicity database. It is important to understand and describe the areas of uncertainty within the toxicity evaluation and derivation of toxicity value (noncancer and cancer slope factor), as use of conservative or “health protective” decisions for addressing each area of uncertainty may result in compounded conservatism, and, therefore, indications of risk may not be realistic. For 1,4-dioxane, the most important uncertainty that may warrant discussion is the cancer MOA and quantitative impact that decision has on the risk assessment. Section 5.2.3.4 provides further details.
The exposure assessment of a site risk assessment involves characterizing the exposure setting, identifying relevant exposure pathways, and quantifying the magnitude, frequency, and duration of potential human exposure to chemicals in environmental media so that estimates of the intake (dose) of the chemical can be derived for each exposure pathway (drinking water, air, contaminated soil, etc.). A risk assessment may use default exposure factors (e.g., how many liters of drinking water consumed by a child or adult), point estimates of site-specific exposure concentrations (e.g., what is the concentration of the contaminant in drinking water), or probabilistic methods to describe the range of scientifically supported estimates of intake (the dose). More background on the performance of exposure assessments is detailed in other guidance [(USEPA 1989); (ITRC 2015)] and is not repeated here. A common approach for addressing uncertainty in the exposure assessment is to apply conservative assumptions to help ensure that risk estimates are protective of most potential receptors, such as assuming an adult drinks 2 liters (about a half-gallon) of contaminated water from a single source every day. This approach is intended to introduce a protective bias to ensure that risks are not underestimated. This protective bias is often reflected in the methods for developing reasonable maximum exposures in the exposure assessment. Probabilistic exposure models may refine these estimates with more realistic and predictive parameters. Risk characterizations should fully document and explore the strengths and weaknesses (uncertainties and bias) in the approach used.
The following documents provide useful resources on risk characterization:
- Elements to Consider When Drafting USEPA Risk Characterizations (USEPA 1995a)
- Risk Assessment Guidance for Superfund [(USEPA 1989); (USEPA 1991); (USEPA 2004b)]
- Risk Assessment Forum White Paper: Probabilistic Risk Assessment Methods and Case Studies (USEPA 2014a).
5.4.2 Ecological Risk Characterization
The ecotoxicity of 1,4-dioxane in water and soil is low relative to human health toxicity, and environmental concentrations are typically low in relation to toxicity thresholds. When assessing ecological risk, 1,4-dioxane would not be a risk driver in the ecologically relevant media (surface water, sediment, and soil) at most environmental sites. Except in unusual circumstances, risk characterization of 1,4-dioxane relative to the environment will require only screening-level risk characterization to conclude that ecological risk is unlikely.
Groundwater is not normally an exposure medium of concern for ecological risk assessment as direct exposure to groundwater is not a complete pathway. As 1,4-dioxane is predominantly a groundwater environmental concern, ecological aspects to consider for 1,4-dioxane exposure are limited. There are three scenarios wherein evaluating ecological aspects of 1,4-dioxane in groundwater may be appropriate:
- 1,4-Dioxane impacts to shallow groundwater, where rooting plants may directly contact groundwater.
- 1,4-Dioxane volatilization from groundwater, resulting in vapors potentially affecting burrowing animals. Volatilization from groundwater can be considered negligible except in unique scenarios. In general, inhalation is considered a complete but insignificant pathway in ecological risk assessment except in rare circumstances where protection of burrowing animals is a defined assessment endpoint. Ecological inhalation screening values for 1,4-dioxane are absent. In general, it can be noted that ecological toxicity thresholds would be much higher than equivalent human health thresholds, and the need for specific ecological evaluation a rare occurrence.
- 1,4-Dioxane migration to surface water via emergent groundwater. Evaluated at the screening level via comparison of groundwater concentrations near the point of emergence with aquatic life surface water criteria adjusted by appropriate dilution factors as allowed by state requirements. Note that established groundwater screening levels protective of surface water present in many states are likely to be driven by human health considerations and not by aquatic life.
The development of the CSM should consider whether any of these situations could be complete and of a potential magnitude that may be of concern. If any of these pathways is complete and significant, it would be addressed via screening-level risk characterization, or, if necessary, baseline-level risk characterization.
Ecological risk characterization of 1,4-dioxane exposure will, under most circumstances, terminate at a screening-level risk characterization, and only in rare circumstances would there be a need for a baseline-level risk characterization. Table 5-7 presents specific considerations and best practices for screening-level risk characterization.
Table 5-7. Ecological screening-level risk characterization: issues and best practices
| Screening-Level Risk Assessment |
| Issue: Exclusion criteria and need for ecological risk assessment Summary: Not all 1,4-dioxane release sites will require ecological evaluation. Exclusion criteria can be used in many jurisdictions to exclude evaluation of 1,4-dioxane concerns if certain conditions are met. Best Practices: Follow local state or regional regulations on ecological exclusion criteria. |
| Issue: Absence of widely applied ecological screening-level criteria Summary: There are few widely accepted ecological screening levels for 1,4-dioxane, and 1,4-dioxane is seldom included in listings of constituents of ecological concern, as discussed in Section 5.3. In many jurisdictions, for contaminants with no generally accepted or prescribed screening level, development of a screening level may be requested. Best Practices: The available ecological screening levels are higher than the corresponding human health criteria, and generally higher or much higher than environmental concentrations. For media or exposures without screening levels, practitioners should consider if environmental concentrations are high enough to potentially be a concern, considering likely toxicity thresholds in other media. Practitioners should also consider whether the need for screening may also become moot if human health concerns are expected to drive risk management decisions. |
| Issue: Complete and significant pathways Summary: 1,4-Dioxane is primarily a groundwater contamination concern of concern for human health. Ecological exposures to toxicologically meaningful concentrations in ecologically relevant media are rare. Best Practices: The development of the CSM should consider if and where ecological exposures could become risk drivers for the site. Such situations may arise in situations where 1,4-dioxane is present in surface water, sediment, or soil where there is no human health exposure pathway. Practitioners should consider the media concentrations in relation to ecotoxicologically relevant concentrations to evaluate which exposures could be a concern. Where 1,4-dioxane is primarily a groundwater concern, practitioners should evaluate if any ecologically relevant exposure pathway exists, such as emergence into surface water, inhalation by burrowing animals, or plant root exposure. Absent these concerns, an ecological risk evaluation is unlikely to be needed. |
5.4.3. Uncertainty Analysis
Uncertainties are integral to all risk assessments, and particularly so for ecological risk assessment considering the variability of real-world ecological systems and the unknowns inherent in evaluating a substance with limited ecotoxicological information, such as 1,4-dioxane. In the uncertainty section, the risk characterization results are evaluated in terms of confidence in the outcome based on the uncertainties, confidence levels, and applicability of the CSM, exposure assumptions, toxicity assumptions, and underlying data. The evaluation of uncertainties can be narrative and may include qualitative or quantitative sensitivity analysis based on alternate assumptions to bound the estimates. Table 5-8 summarizes key uncertainties in the risk assessment of 1,4-dioxane.
Table 5-8. Effect of uncertainties on human health and ecological risk characterization specific to 1,4-dioxane
| Key Uncertainty Issue | Potential effect on risk estimates (overpredict/ underpredict) | Typical magnitude human health | Typical magnitude ecological |
| Completeness of CSM | Underpredict | Small to moderate. There may be assumptions and data gaps regarding dermal and inhalation risk that may apply. | Small. There are limited ways for ecological 1,4-dioxane exposure at toxicologically relevant concentrations. |
| Sample and site spatial and temporal heterogeneity | Underpredict | Small. Analytical methods for 1,4-dioxane in various relevant media are available and are typically sensitive enough to produce high-quality data to characterize a given site. | Small. 1,4-Dioxane concentrations in ecologically relevant media are typically relatively homogeneous and low relative to toxicologically relevant concentrations. |
| Derivation of toxicity values | Overpredict | Moderate to large. Using a low-dose linear extrapolation for 1,4-dioxane’s cancer evaluation likely overestimates cancer risks given what is understood about its cancer MOA [(HC 2018); (Dourson et al. 2017); (USEPA 2013b); (USEPA 2014a)]. | Small to moderate. Ecotoxicity tests are carried out using methods designed to maximize bioaccessibility and exposure. |
| Screening levels | Overpredict | Large. There may be several orders of magnitude difference in screening level, depending on the choice of toxicity value and exposure assumptions. Exposure estimates for default screening levels typically overestimate exposures (e.g., assume a human drinks 2.5 L of water a day for their lifetime). Environmental concentrations of 1,4-dioxane in groundwater, for example, generally fall within the calculated range of possible screening levels. | Small in practice, as environmental concentrations are generally much lower than applicable screening levels. |
| Bioavailability | Not likely important for 1,4-dioxane | Not applicable for human health, as 1,4-dioxane is readily absorbed through ingestion of contaminated water, and, if present, through inhalation. | Small in practice, as environmental concentrations are generally much lower than toxicologically relevant concentrations, and differences in site-specific bioavailability have no substantial effect. |
5.5 Risk Communication
USEPA’s Risk Communication Guidance (USEPA 2007) mentions that the purpose of risk communication is threefold:
(1) Assist affected communities in understanding risk assessment.
(2) Assist affected communities in forming perceptions of the potential hazards.
(3) Assist affected communities in making decisions about how risk should be managed.
Risk communication can potentially be difficult at times as environmental hazards of emerging contaminants, such as 1,4-dioxane, often have incomplete scientific data and the science is rapidly evolving. It is very important that risk communicators be able to gain the trust of affected communities and relay messages that are transparent and easily understandable for communities. ITRC has developed a Risk Communication Tool Kit that highlights the value of a science-based communication approach when providing people information about potential hazards and discusses risk communication challenges when addressing emerging contaminants. This document also includes engagement methods and tools, as well as case study examples of how to improve risk communication activities (see the ITRC Risk Communication document).
NJDEP Comments on 1,4 Dioxane Toxicity and Risk Assessment
The New Jersey Department of Environmental Protection recently issued comments that pertain to this specific document section. These comments were provided after the 1,4 Dioxane document completed external review and was published. These comments are available in a full PDF of this section.