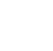3. Environmental Fate, Transport, and Investigative Strategies
This section of the guidance document provides an overview of the chemical and physical properties of 1,4-dioxane and discusses the fate and transport processes in the context of these properties. This section also provides a framework for developing conceptual site models (CSMs) for 1,4-dioxane and a summary of relevant site investigation strategy considerations that may be relevant under various regulatory frameworks (e.g., CERCLA, RCRA, and state cleanup programs).
The fate and transport of 1,4-dioxane following its release to the environment is a function of key chemical and physical properties of the compound as well as environmental characteristics. 1,4-Dioxane’s chemical and physical properties help us understand what processes (discussed below) are likely to be relevant in influencing its distribution in the environment and the potential risk associated with its migration. For example, a defining characteristic for 1,4-dioxane is its low sorption potential (low organic carbon partitioning coefficient), which makes it mobile in groundwater and has led to concerns that 1,4-dioxane plumes will expand beyond those of other common co-contaminants like CVOCs. In addition, based on it’s aerobic degradation pathways characterized to date, 1,4-dioxane is not expected to be attenuated via the same pathways that have shown to be relevant for other co-contaminants (e.g., anaerobic reductive dechlorination of chlorinated solvents). However, some recent evidence suggests that anaerobic 1,4-dioxane biodegradation may be possible [(Ramalingam and Cupples 2019); (Shen, Chen, and Pan 2008)]. Site environmental characteristics will also influence how 1,4-dioxane behaves following its release to the environment, determining where transport will occur and at what rate. Additionally, 1,4-dioxane’s mobility may increase the importance of cross-media transfer (e.g., groundwater to surface water) and other issues relative to less mobile contaminants.
The information in this section can be used as a framework to support the development of a site-specific CSM that defines how these processes influence 1,4-dioxane within a particular environmental setting. The CSM should be used to identify areas of current and future concern, as well as evaluate exposure pathways for potential receptors. The goal is to provide a more informed basis for 1,4-dioxane site investigation and help ensure that investigation strategies prioritize any knowledge gaps. The properties of 1,4-dioxane also strongly influence the potential effectiveness of any treatment or remediation technology, and thus a clear understanding of 1,4-dioxane fate and transport is critical to remedial decision-making and long-term management strategies. As a result, the material in this section also helps support the technical basis for the subsequent section on treatment and remediation of 1,4-dioxane (Section 6).
3.1 Chemical and Physical Properties
Common 1,4-dioxane identifiers are summarized in Appendix B. Physical and chemical properties of 1,4-dioxane are summarized in Appendix B. The key properties that are relevant to 1,4-dioxane’s fate and transport include:
- 1,4-Dioxane’s low Henry’s law constant indicates a low potential for volatilization in the liquid phase compared to other solvents, but its high vapor pressure indicates potential for volatilization from dry surfaces. It is worth noting that the azeotropic properties of 1,4-dioxane cause volatility to increase significantly with temperature; however, this occurs only at temperatures well above what is typical in the environment.
- 1,4-Dioxane has a low organic carbon partitioning coefficient (Koc), which results in low affinity for organic carbon, and is also nonionic (e.g., uncharged). As a result, 1,4-dioxane does not bind strongly to soils or sediment and readily leaches out of soils into groundwater or surface water.
- 1,4-Dioxane’s density is close to that of water, and it is fully miscible.
These characteristics of 1,4-dioxane control its partitioning in media and the relative importance of fate and transport processes.
Table 3-1 compares the chemical and physical properties of 1,4-dioxane with those of commonly co-occurring CVOCs at sites of environmental concern. As a result of differences in these compounds’ fundamental properties, they are expected to behave distinctly in the subsurface, as discussed in greater detail in Sections 3.2.
Table 3-1. Comparison of relative physical and chemical properties of 1,4-dioxane with other chemicals
| Property | Units | 1,4-Dioxane* | Water | Benzene | TCE | 1,1,1-TCA | 1,1-DCA | 1,1-DCE |
| CAS registry number (CASRN) |
— | 123-91-1 | 7732-18-5 | 71-43-2 | 79-01-6 | 71-55-6 | 75-34-3 | 75-35-4 |
| Molecular formula |
— | C4H8O2 | H2O | C6H6 | C2HCl3 | C2H3Cl3 | C2H4Cl2 | C2H2Cl2 |
| Molecular mass |
g/mol | 88.11 | 18.02 | 78.11 | 131.4 | 133.4 | 98.96 | 96.94 |
| Boiling point | °C at 1 atm | 101 | 100 | 80 | 87.2 | 74 | 57.4 | 31.7 |
| Water solubility |
g/liter at 25°C | 1,000 | — | 1.79 | 1.28 | 0.91 | 5.04 | 2.42 |
| Vapor pressure |
mm Hg at 25°C | 38.1 | 23.8 | 94.8 | 69 | 124 | 227 | 634 |
| Henry’s law constant (in water) |
Atm-m3/mol at 25 °C | 4.8 × 10-6 | — | 5.55 × 10-3 | 9.85 × 10-3 | 1.6 × 10-2 | 5.62 × 10-3 | 2.61 × 10-2 |
| Log Koc | Dimensionless | 0.54 | — | 1.75 | 2.0 | 1.95 | 1.55 | 1.85 |
| Log Kow | Dimensionless | –0.27 | — | 2.13 | 2.42 | 2.49 | 1.79 | 2.13 |
*1,4-Dioxane forms an azeotrope with water in which 48.5 mole percent of 1,4-dioxane in water will boil at 87.59°C. Source: Estimation Programs Interface Suite [(USEPA and SRC 2019); (USEPA 2019c)].
g/mol: grams per mole; °C: degrees Celsius; mm Hg: millimeters of mercury; atm-m3/mol: atmosphere-cubic meters per mole.
3.1.1 Volatilization
USEPA and many states classify chemicals as volatile based on both their Henry’s law constant and their vapor pressure at room temperature (25°C) [(ITRC 2007); (USEPA 2015a)]. Volatile chemicals are generally defined as those with a Henry’s law constant greater than 1 x 10-5 atm-m3/mole or a vapor pressure greater than 1 millimeter of mercury (mm Hg) (USEPA 2015a). 1,4-Dioxane has a vapor pressure of 38.1 mm Hg, which meets this definition for volatility, and a Henry’s law constant of 4.8 x 10-6 atm-m3/mole for water, which does not meet this definition. The high vapor pressure and low Henry’s law constant indicate 1,4-dioxane volatilization is a moisture-dependent process. The vapor pressure indicates 1,4-dioxane will readily volatilize from a dry soil source (e.g., a release from an industrial-grade product where there is insufficient moisture to dissolve the 1,4-dioxane), but the Henry’s law constant indicates 1,4-dioxane volatilization from water is unlikely to occur. Mackay’s fugacity modeling confirms this assumption; in the presence of moisture and at ambient environmental temperatures, the model predicts a significant partitioning of 1,4-dioxane into the water phase (i.e., 90% water, 9% air, 1% soil and negligible amounts in sediments) (Environment Canada 2010). Thus, 1,4-dioxane is not typically considered a compound of interest for vapor intrusion (VI) when dissolved in water but may warrant further VI consideration when present under dry soil conditions.
The Henry’s law constant is temperature dependent, and the degree of volatility will also be influenced by the level of moisture content in the soil. 1,4-Dioxane forms a positive azeotrope with water, which may enhance partitioning to the vapor phase when applying in situ thermal treatment methods where the temperature is increased to the boiling point of 1,4-dioxane/water mixtures (see Section 6.5.2.4). However, this is unlikely to be relevant at ambient environmental temperatures.
Notably, there is limited information on the transport of 1,4-dioxane in the vadose zone, as well as its occurrence in indoor air due to VI. This may be related to the analytical challenges or because 1,4-dioxane hasn’t been viewed as a potential VI concern. In recent presentations on the topic [(Holton, DiGuiseppi, and Mohr 2017); (Bell et al. 2019)], practitioners have cited the limited data on this subject and the likelihood that other comingled compounds are more likely to drive the VI risk unless certain conditions are met (e.g., relatively dry soils, shallow vadose zone sources, and infiltrating contaminated groundwater).
3.1.2 Partitioning to Solid-Phase/Adsorption
The extent to which contaminants partition from the aqueous phase (e.g., groundwater) to the solid phase (e.g., soil particles) has the potential to affect transport. This can occur via several mechanisms in a groundwater-bearing unit, but sorption is generally the process of primary interest for organic, uncharged (e.g., nonionic) contaminants. Sorption occurs due to the hydrophobic properties of many organic contaminants, such that they prefer to associate with the organic carbon-rich portions of a soil or sediment particle rather than water. In the case of a hydrophilic compound like 1,4-dioxane, the tendency to sorb to solids is low. This is reflected in the reported values for partitioning coefficient, including the octanol-water partitioning coefficient (Pankow and Cherry) and Koc (see Appendix B). The latter coefficient is the ratio of the soil to groundwater concentration at equilibrium (after correcting for the organic carbon content in the soil) and is particularly relevant for modeling fate and transport in aquifers. The 1,4-dioxane Koc values are one or more orders of magnitude lower than potentially co-occurring chlorinated solvents like 1,1,1-TCA or TCE.
Based on reported Koc values, estimates of the retardation factor for 1,4-dioxane—the ratio of the groundwater velocity to the contaminant velocity—are likely to be close to 1. As a result, 1,4-dioxane’s migration is not expected to be significantly retarded relative to the groundwater velocity. The concept of retardation can also be used to establish potential travel times and plume expansion rates for 1,4-dioxane and any contaminants based on advective flow, as discussed in Section 3.2.3.
The low sorption potential for 1,4-dioxane is also relevant to transport within the unsaturated zone. Because 1,4-dioxane sorption to organic carbon is limited, and it lacks a charge that would facilitate electrostatic interactions, retention within the vadose zone is generally expected to be limited. As a result, 1,4-dioxane released to soil would be expected to readily leach to groundwater, at rates that depend on infiltration rates than on the soil’s organic carbon content.
3.1.3 Advective Transport
1,4-Dioxane that has entered groundwater or surface water is subject to further migration due to the advection process. Advection refers to the movement of a compound within a fluid that is flowing in response to changes in pressure, density, or elevation. In the case of an aquifer where the transport of 1,4-dioxane is being evaluated, advection describes the movement of groundwater due to changes in hydraulic head (i.e., gradient). Advection is a function of the geological media properties, including the effective porosity and hydraulic conductivity. The contaminant’s properties can determine its transport relative to the normal advective flow of groundwater. This can include partitioning to solid phases (Section 3.1.2), diffusion (Section 3.1.4), and dispersion.
Advection is a relevant transport process for all contaminants. However, it is a particularly important consideration for 1,4-dioxane due to the contaminant’s relatively low tendency to partition to soil (see Section 3.1.2). As a result, 1,4-dioxane can migrate rapidly within an aquifer, potentially at a similar velocity to groundwater. This would lead to the potential for rapid plume expansion in groundwater systems that flow at significant velocities due to steep hydraulic gradients and highly permeable soils.
To understand the potential for 1,4-dioxane migration in groundwater (e.g., travel distance) due to advection, we evaluated a hypothetical release scenario for 1,4-dioxane (Figure 3-1). In this example, we assumed that 1,4-dioxane was released along with 1,1,1-TCA in 1970, a period when 1,1,1-TCA use as a solvent was relatively common (see Section 1). The transport of 1,4-dioxane along a one-dimensional flow path was modeled along with 1,1,1-TCA and its typical by-products (1,1-DCE and 1,1-DCA). In this case, the release occurred in an aquifer with a range of organic carbon contents to incorporate the retardation effects due to solid-phase partitioning. At this same location, we also assumed a release of TCE to have occurred in 1955, which is consistent with TCE’s historical usage history as an industrial solvent. In both cases, we assumed that 1,4-dioxane is not degraded and that CVOC degradation occurs only within the source area.
The results of this exercise show that the expected plume size for 1,4-dioxane falls within a similar range as TCE and one of its possible degradation products (1,1-DCE). In addition, 1,4-dioxane’s plume size was predicted to be comparable to the 1,1-DCE and 1,1-DCA plume sizes from the 1970 release. This is largely because the retardation factors for several of these common co-contaminants (particularly 1,1-DCE) are very similar to 1,4-dioxane (i.e., close to 1) in an aquifer with typical organic carbon content. Furthermore, at sites where the use of multiple solvents is suspected, releases of TCE (and its likely degradation products) from earlier periods provides a head start that is relevant in the context of advection. These results are consistent with the plume size data reported in (Adamson et al. 2014b), which showed that chlorinated solvent plumes were typically the same size or longer than colocated 1,4-dioxane plumes.
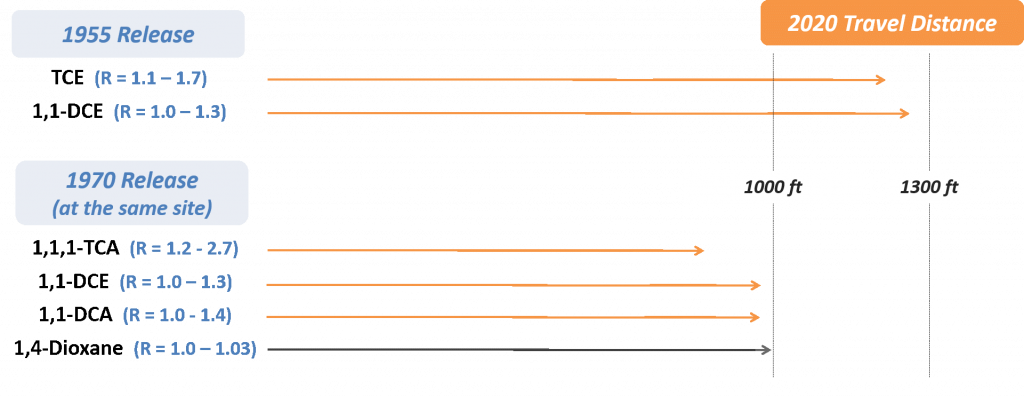
Figure 3-1. Evaluation of Travel Distance for 1,4-Dioxane and Common Co-Contaminants Based on Hypothetical Release Scenario.
Shaded bars shown the range of travel distances for each compound based on one-dimensional advective transport in a hypothetical groundwater system with a seepage velocity of 20 ft/yr. For each contaminant, the travel distance is the product of the time since release (in years), the seepage velocity, and the contaminant specific retardation factor (R).
The retardation factor is equal to ![]()
Values of 1.67 g/cm3 and 0.3 were used for the soil bulk density and porosity, respectively, along with log Koc values in Appendix B (including log Koc = 0.54 for 1,4-dioxane). The range of retardation factors (R) are based on high (0.002) and low (0.0002) input values for the fraction of organic carbon (fOC), which were used to calculate the range of travel distances represented by the shaded bars. Calculation does not include effects of dispersion/diffusion, and it assumes that no 1,4-dioxane degradation occurs and that CVOC degradation occurs only in the source area.
Source: ITRC 1,4-Dioxane Team, 2020.
The hypothetical release scenario described above emphasizes the importance of quantifying the groundwater (seepage) velocity and flow direction to understand 1,4-dioxane transport at contaminated sites (see the ITRC Mass Flux document). These are required input parameters for fate and transport modeling of 1,4-dioxane, and understanding how these flow characteristics vary across a site will result in fate and transport modeling that better predicts plume behavior (particularly for large plumes). This could include changes in the magnitude or direction of groundwater flow along the flow path in response to variations in temperature, pumping, or other factors. Consequently, proper characterization of the flow system should be an important component when developing a CSM for 1,4-dioxane and developing a site characterization strategy.
Dispersion can also occur within the subsurface and may contribute to spreading of 1,4-dioxane in some cases, but contaminant dispersion is likely to be a minor process in groundwater relative to advection (Payne, J.A., and S.T. 2008). Dispersion may be more important for understanding transport of 1,4-dioxane releases to surface water or air due to the nature of those matrices and their associated flow characteristics.
3.1.4 Matrix Diffusion
Matrix diffusion is a term used to describe the diffusion of contaminants both into and out of an aquifer’s lower-permeability zones (e.g., clays, silts, bedrock) [(Feenstra, 1984 #231); (Sale et al. 2013)]. It has been increasingly recognized as a contributor to source and plume longevity for chlorinated solvents [(Liu and Ball 2002); (Chapman and Parker 2005); (Parker, Chapman, and Guilbeault 2008); (Leeson, Stroo, and Johnson 2013)], but it only recently received attention as an important process for 1,4-dioxane (Adamson et al. 2016). The concept of matrix diffusion is based on natural molecular diffusion, where compounds move in response to a concentration gradient (i.e., diffusing from a high-concentration area to a low concentration area). As a result, all contaminants will diffuse, but the rate of diffusion in groundwater is generally considered a slow process. Rates are largely defined by the molecular diffusion coefficient of the contaminant in water, and the value reported for 1,4-dioxane (0.32 – 2.38 x10-5 cm2/s; see Appendix B) is similar to its common co-contaminant 1,1,1-TCA (8.8 x 10-5 cm2/s). Diffusion is also a function of aquifer properties, specifically the tortuosity (Pankow and Cherry 1996). Diffusion is applicable in all subsurface environments, but it is of particular interest in aquifers where soils of highly contrasting permeabilities (e.g., sands and clays) are in close contact. This is because any contaminant mass that diffuses into lower-permeability soils will be subject to slower advection and thus become more persistent (see Figure 3-2).
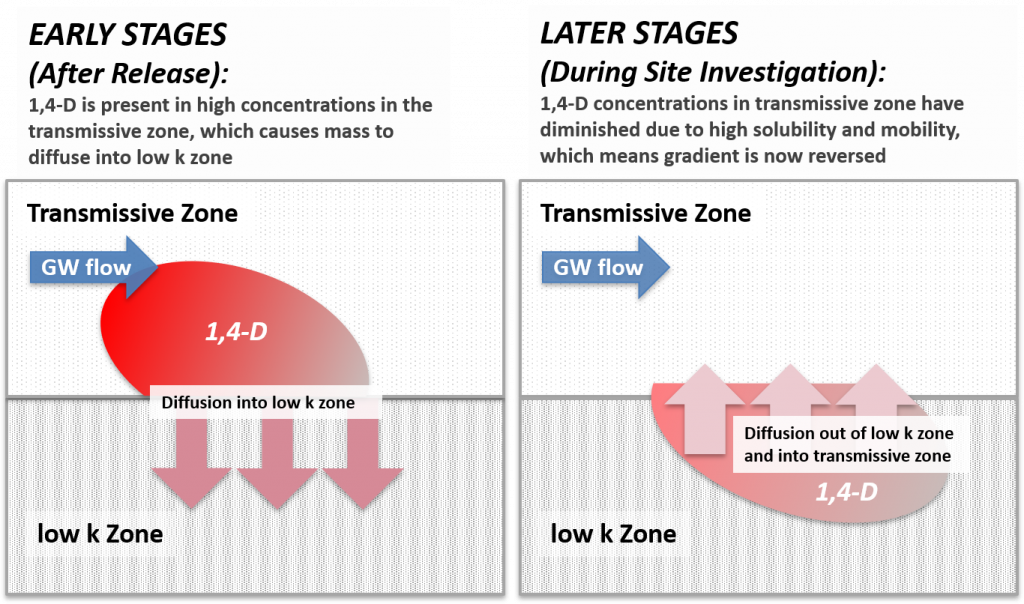
Figure 3-2. Overview of Matrix Diffusion Process for 1,4-Dioxane. The left panel represents the early stages following a release, when diffusion into a lower-permeability (low k) zone may occur due to high 1,4-dioxane concentrations in the transmissive zone. The right panel represents the later stages, when a change in the concentration gradient results in back-diffusion of 1,4-dioxane into the transmissive zone.
Source: ITRC 1,4-Dioxane Team, 2020.
Matrix diffusion has the potential to increase persistence of 1,4-dioxane in the subsurface for several reasons. First, the high effective solubility of 1,4-dioxane creates an initial concentration gradient that can drive large amounts of mass into lower-permeability zones. Second, 1,4-dioxane sorbs poorly, meaning that significant penetration into a low-permeability layer can occur because there is less resistance to diffusion-driven transport. Conceptually, it is expected that 1,4-dioxane will move rapidly through highly transmissive portions of the aquifer, but it will invade less permeable materials by diffusion.
Back-diffusion of 1,4-dioxane mass from these less permeable zones might serve as a dominant long-term secondary source [(Adamson et al. 2016); (Mackay and Cherry 1989)]. A recent modeling study examined a typical release scenario for 1,4-dioxane and 1,1,1-TCA to demonstrate the potential impact of matrix diffusion processes (Adamson et al. 2016). Significant storage of 1,4-dioxane mass in a low-permeability clay underlying a transmissive sand layer was predicted to occur and contribute to long-term persistence of a 1,4-dioxane plume. The 1,4-dioxane source dissolved rapidly and a significant portion of the mass migrated downgradient, such that the concentration gradient in the source area reversed within a few years. This led to back-diffusion of 1,4-dioxane from the clay into the sand layer, with a source-zone aqueous concentration that was predicted to remain above several hundred µg/L for longer than 80 years after the release. This level was higher than the level predicted for 1,1,1-TCA at the same point in time. Similarly, rapid migration of 1,4-dioxane was predicted to impact a longer downgradient area than 1,1,1-TCA, such that back-diffusion of 1,4-dioxane would be an issue across a larger plume footprint.
Data from surveys of 1,4-dioxane-contaminated sites indicate that the maximum concentrations at these sites are generally in the tens to hundreds of µg/L [(Anderson, Anderson, and Bower 2012); (Adamson et al., 2014a); (Chiang et al. 2016); (Karges, Becker, and Püttmann 2018)]. These levels are reasonably consistent with those predicted to occur by diffusion-driven processes, such that matrix diffusion is potentially a significant contributor to the 1,4-dioxane concentrations that are currently being measured at field sites. Storage of mass in low-permeability soil has been noted in several field investigations of 1,4-dioxane (Adamson, Newell, et al. 2017). At these sites, the back-diffusion of mass from the lower-permeability zone represents a “secondary source” of 1,4-dioxane that is particularly relevant after source material in the transmissive zone has been depleted. This process has several important implications for managing 1,4-dioxane at contaminated sites, including the following:
- At sites where diffusion of 1,4-dioxane is the dominant contributing factor to long-term plume persistence, the release area for 1,4-dioxane may be difficult to identify and delineate. This challenge is exacerbated by 1,4-dioxane’s migration potential in groundwater. At these sites, it may be more efficient to delineate the chlorinated solvent source zone and then use this to approximate the area where 1,4-dioxane was released. Further delineation of areas where diffusion-based “secondary sources” are suspected may also be necessary at such sites.
- Sites where matrix diffusion is active are likely to be harder to clean up using conventional in situ technologies that rely on distribution of amendments through injection. Management of these sites may require alternative approaches such as monitored natural attenuation (Shields-Menard et al.) (see Section 6).
3.1.5 Groundwater-to-Surface Water Discharge
Interaction between groundwater and surface water can lead to cross-media transfer of contaminants and may accelerate overall transport rates. Groundwater discharge to surface water is common and occurs whenever the water table of an aquifer is higher than the elevation of the adjacent surface water body (USGS 1999). These are referred to as gaining streams, and the baseflow represented by groundwater influx may be significant.
In the case of 1,4-dioxane, the primary concern is likely to be the discharge of 1,4-dioxane from contaminated groundwater sites into nearby surface water bodies such as rivers. This process could contribute to further spreading of 1,4-dioxane contamination away from the source due to the rapid advection within many receiving streams and rivers. At some sites, this process could also result in the need for a more comprehensive risk assessment due to the introduction of new pathways and receptors. Some state agencies have cleanup regulations that specifically address the groundwater–surface water interface as a pathway (e.g., (MDEQ 2018)).
For 1,4-dioxane, one potential concern would be any discharge of 1,4-dioxane into surface water that is being used as a source of drinking water. The occurrence of 1,4-dioxane in U.S. drinking water systems that rely on surface water has been established based on data collected in support of UCMR3, with 1,4-dioxane detected in 10% of drinking water samples from surface water sources (Adamson, Piña, et al. 2017). However, the extent to which groundwater-to-surface water discharge was responsible for the detected 1,4-dioxane relative to direct discharge of 1,4-dioxane from other sources is not clear. One potential additional source of 1,4-dioxane in surface water is discharge from WWTPs (see Section 1.3.1). These facilities may receive 1,4-dioxane impacted influent, which may be inadequately treated prior to surface water discharge.
Given the mobility of 1,4-dioxane in groundwater, the potential discharge of this contaminant from groundwater sources to surface water should be evaluated on a site-by-site basis. The mass of 1,4-dioxane discharged is a function of the 1,4-dioxane concentration in groundwater and the flow rate of groundwater. The surface water 1,4-dioxane concentration may be dilute in cases where the receiving stream flow rate is high relative to the flow rate of groundwater. In addition, dissolved oxygen is typically introduced into shallower intervals near the groundwater–surface water interface (Lendvay et al. 1998), and because 1,4-dioxane is biodegraded through aerobic pathways (Section 3.1.7), this process may increase the natural attenuation capacity near the point of discharge.
3.1.6 Photodegradation
In the atmosphere, 1,4-dioxane does not undergo direct photooxidation, meaning that 1,4-dioxane itself does not absorb ultraviolet (UV) radiation. This is because it lacks functional groups that absorb light in the UV range of natural sunlight (Lyman, Reehl, and Ronsenblatt 1982). Indirect photooxidation occurs when energy is transferred from a radical species formed by UV light and results in degradation. 1,4-Dioxane is subject to indirect photooxidation, and the primary mechanism is reaction with hydroxyl radicals (•OH) formed in the presence of UV light. This process is expected to start within several hours of UV light and •OH exposure, and its rate is dependent on •OH concentrations, with a reported second-order rate constant of 10.9 x 1012 cm3/molecule-sec (Atkinson 1989). Based on the equation below, this rate constant would result in atmospheric half-lives for 1,4-dioxane of between 1.0 and 2.9 days for •OH radical concentrations between 0.5 x 106 and 1.5 x 106 •OH radicals/cm3 and a 12-hour day (ATSDR 2012).
t1/2 = 1/k[A0]
t1/2 = half-life
k = second-order rate constant
A0 = starting concentration (•OH)
As a result of indirect photooxidation, releases of 1,4-dioxane to air are not likely to cause environmental issues. Best available information currently does not provide documented evidence of natural photodegradation of 1,4-dioxane in surface water. Direct photolysis in surface water is not expected (based on expected light intensity, the angle of light entry, turbidity, and dissolved organics in water) (Mohr et al. 2020). Any potential indirect photodegradation of 1,4-dioxane in surface water may be impacted by other constituents that compete for UV light, such as dissolved organic carbon and nitrates.
3.1.7 Biodegradation
Biodegradation is the microbially mediated transformation of a substance into separate components via biochemical reactions. Microorganisms use a variety of organic contaminants as carbon and energy sources by performing oxidation-reduction reactions that transfer electrons from one constituent to another (Madigan et al. 2015). Biodegradation via oxidation is the primary known destructive process for 1,4-dioxane in the subsurface. This process occurs aerobically (i.e., with oxygen). In this process, electrons are transferred from 1,4-dioxane (electron donor) to oxygen (electron acceptor). In addition to performing this process to gain carbon and energy (metabolism), microorganisms also cometabolize 1,4-dioxane during the oxidation of other organic compounds. Further descriptions of metabolic and cometabolic 1,4-dioxane biodegradation are included in Section 3.1.7.1 and Section 3.1.7.2, respectively.
The microorganisms mediating 1,4-dioxane biodegradation processes are bacteria (McElroy and Hyman 2019) and fungi [(Nakamiya et al. 2005); (Kinne et al. 2009)]. The first step in currently known bacterial 1,4-dioxane biodegradation pathways is mediated by a variety of monooxygenase enzymes, although not all monooxygenase enzymes degrade 1,4-dioxane [(Mahendra and Alvarez-Cohen 2006); (McElroy and Hyman 2019)]; and references therein). Enzymes are biological molecules that catalyze chemical reactions. Microorganisms produce enzymes to perform the chemical reactions that they rely on for growth and reproduction. The function of monooxygenase enzymes depends on the availability of oxygen, although they may remain active even at low oxygen concentrations. Anaerobic 1,4-dioxane biodegradation (biodegradation without oxygen, but with alternative electron acceptors) has been investigated, and there is some laboratory evidence that this process may occur [(Ramalingam and Cupples 2019); (Shen, Chen, and Pan 2008)]. However, degradation pathways and relevant microorganisms have not been characterized. Furthermore, evidence of anaerobic 1,4-dioxane biodegradation in the natural environment has not been published. To date, the role of fungi in 1,4-dioxane biodegradation at contaminated sites has undergone limited investigation.
Intermediate products have been identified in multiple degradation pathways [(Mahendra et al. 2007); (Huang et al. 2014); (Chen et al. 2016); (Deng, Li, and Li 2018)]. In general, 1,4-dioxane biodegradation intermediates are innocuous products that are readily biodegraded. These intermediates are not expected to accumulate in the environment, are not common laboratory analytes, and generally do not have regulatory criteria.
3.1.7.1 Metabolic biodegradation
When the transformation of a constituent results in carbon and/or energy yield to the microorganism, the process is termed “metabolic biodegradation.” Researchers have identified microorganisms capable of aerobic metabolic 1,4-dioxane biodegradation [(Mahendra and Alvarez-Cohen 2005); (Kim et al. 2009); (McElroy and Hyman 2019)]; and references therein). However, it is not known if there are additional species that may mediate this process in the environment. The relative abundance of microorganisms capable of metabolic 1,4-dioxane biodegradation in the environment is unknown.
In general, if oxygen is present, metabolic 1,4-dioxane biodegradation rates are expected to depend on 1,4-dioxane concentrations (first-order kinetics). However, robust metabolic biodegradation is likely only to be supported at 1,4-dioxane concentrations sufficient to meet energy demands, even under favorable environmental conditions. The 1,4-dioxane substrate concentrations needed to support microbial growth are likely in the range of hundreds of micrograms to milligrams per liter (Barajas-Rodriguez et al. 2019). If concentrations of 1,4-dioxane in groundwater are less than the amounts necessary for microbial growth, metabolic biodegradation may not occur at meaningful rates.
3.1.7.2 Cometabolic Biodegradation
In addition to metabolic biodegradation processes, microorganisms may also degrade constituents, including 1,4-dioxane, as a side effect of degradation of a different constituent that they are targeting for carbon and/or energy (primary substrates). This process is termed “cometabolism” [(Horvath 1972); (McCarty 1987); (Hazen 2019)]. Cometabolism is the result of enzymes that have a tendency to bind to and act on both target compounds (primary substrates) and other compounds that happen to co-occur (cometabolic substrates). Microorganisms do not have the capability to harvest carbon or energy from compounds whose enzymes cometabolically degrade because they do not make the additional enzymes needed to perform subsequent reactions in the degradation pathway or do not have a pathway to assimilate intermediate breakdown products. Tetrahydrofuran (THF), propane, toluene, n-butane, n-pentane, isobutane, isopentane, and ethane have been identified as primary substrates that can support 1,4-dioxane cometabolism [(Mahendra and Alvarez-Cohen 2006); (McElroy and Hyman 2019); (Lan, Smith, and Hyman 2013); Rolston, Hyman, and Semprini 2019); (Hatzinger et al. 2017); (Xiong et al. 2020)]. Methane-linked cometabolic biodegradation has also been reported (Mahendra and Alvarez-Cohen 2006); however, demonstration of this process has not been consistently successful (Hatzinger et al. 2017).
Because cometabolic degradation does not provide a source of carbon or energy to the microorganisms that mediate the reaction, the reaction is controlled by the availability of the primary growth substrate(s). In the cometabolic case, 1,4-dioxane degradation rates depend on the availability of the primary substrate and oxygen, and both relative to 1,4-dioxane. If primary substrate concentrations are elevated, substrate inhibition is likely to limit 1,4-dioxane degradation (Alvarez-Cohen and Speitel 2001). In this case, substrate inhibition is the slowing or halting of cometabolic biodegradation as a result of enzymes being fully used to target the primary substrate, as a result of its abundance and utility for growth. When a sufficient concentration of primary substrate is available to support microbial growth, and the concentration of 1,4-dioxane is also relatively elevated, then robust cometabolic attack of 1,4-dioxane is likely.
Cometabolic 1,4-dioxane biodegradation may be an important control on fate and transport of 1,4- dioxane where relevant primary substrates are available [(Gedalanga et al. 2016); (Hatzinger et al. 2017); (Chiang et al. 2016)]. This may be particularly relevant in groundwater downgradient of reductive dechlorination of chlorinated ethanes that 1,4-dioxane often co-occurs with (e.g., 1,1,1-TCA). This is because degradation of these constituents produces ethane, which is an effective cometabolic substrate [(Hatzinger et al. 2017); (Xiong et al. 2020)]. Ethane may facilitate 1,4-dioxane biodegradation in redox transition zones, at the boundary between oxic zones—where oxygen is available—and anoxic zones—where ethane is produced via reductive dechlorination.
3.1.7.3 Inhibitory Compounds
In addition to being influenced by concentrations of 1,4-dioxane, primary substrates, and oxygen, biodegradation potential may be influenced by the presence of common chlorinated co-contaminants and metals. This influence may include inhibition via toxicity to the degrading microbe, inhibition due to interference with enzyme or other cellular activity, or inhibition due to substrate competition (Alvarez-Cohen and Speitel 2001). Substrate competition results from monooxygenase enzymes cometabolically attacking co-contaminants other than 1,4-dioxane, potentially as a result of greater abundance and therefore greater likelihood of contact between the enzyme and the alternate cometabolic substrate. This inhibition mechanism is particularly relevant where dissolved oxygen is limited. Inhibitory effects of potential co-contaminants and metals have been investigated for a subset of microorganisms that metabolically or cometabolically degrade 1,4-dioxane [(Mahendra, Grostern, and Alvarez-Cohen 2013); (Zhang, Gedalanga, and Mahendra 2016); (Inoue et al. 2020); (Zhao et al. 2018)]. Findings from these studies suggest that 1,1-DCE and copper (II) have substantial potential to inhibit 1,4-dioxane biodegradation and that 1,1,1-TCA, TCE, and cis-1,2-DCE also inhibit at least some 1,4-dioxane-degrading organisms under some conditions. Relative concentrations of 1,4-dioxane and potential inhibitory substrates are likely an important control on the degree of potential inhibition. However, other factors, including availability of oxygen, whether degradation is metabolic or cometabolic, and which microorganisms are responsible for degradation, are also important controls. Currently, there is not an established predictive capacity for which in situ conditions (e.g., potential inhibitory compound concentrations) may result in sufficient inhibition to affect the efficacy of biodegradation in meeting remediation objectives.
3.1.7.4 Site Investigation of Biodegradation
Meaningful 1,4-dioxane biodegradation will result in measurable mass loss that is greater than that expected from physical attenuation processes. Therefore, a preliminary assessment of biodegradation may involve assessing mass flux or 1,4-dioxane concentrations over distance and time to determine if there is an apparent loss. In cases where there is evidence of contaminant destruction, additional lines of evidence may be developed to determine likely biodegradation mechanisms. Additional lines of evidence may include demonstration of geochemical conditions that are conducive to biodegradation (e.g., sufficient oxygen concentrations), demonstration that relevant microorganisms are present and active, and application of compound-specific isotope analysis (CSIA) to evaluate the degree of isotope fractionation attributable to biodegradation. These lines of evidence are discussed in additional detail in the Subsection 6.5.2.1. Advanced analytical methods (including application of molecular biology tools and CSIA) are discussed in the Subsection 4.2.4. In some cases, it may be possible to estimate degradation rates at either the bench or field scale. The degree to which these rate estimates are representative of site conditions depends strongly on appropriate experiment design.
3.2 Considerations for Developing or Refining a CSM for 1,4-Dioxane
As discussed in Section 3.2 of ITRC’s RISK-3 guidance (ITRC 2015), CSMs are important tools for addressing environmental problems. By organizing and integrating both site-specific and general physical and chemical data, the CSM helps develop a holistic understanding of the fate and transport of environmental contaminants as well as the potential risk posed by these compounds. Given the physical and chemical properties of 1,4-dioxane, once potential release mechanisms and potentially impacted media have been identified, the fate and transport processes described above provide a framework for developing and refining the CSM through investigation. Specifically:
- The physical properties of 1,4-dioxane imply that this constituent has a greater propensity to enter and remain in groundwater and may be subject to significant matrix diffusion and more rapid advection than other co-occurring constituents.
- Known 1,4-dioxane biodegradation processes are aerobic (Zhang, Gedalanga, and Mahendra 2017) and are therefore distinct from the anaerobic processes often relied on for in situ remediation of colocated chlorinated organics.
- End products of anaerobic biodegradation of other organics (e.g., ethane) may facilitate 1,4-dioxane biodegradation in redox transition zones (Hatzinger et al. 2017).
These 1,4-dioxane fate and transport attributes, as well as details regarding the history of use, highlight how a 1,4-dioxane investigation strategy may be influenced by site-specific characterization efforts for co-occurring contaminants that may have already been performed. In addition to source characteristics and the chemical and physical properties of 1,4-dioxane, site-specific geological and hydrological characteristics are important determining factors in this constituent’s fate and transport behavior. Figure 3-3 shows a generalized CSM for 1,4-dioxane in the environment.
Figure 3-3. Fate and transport processes for 1,4-dioxane releases to the environment.
Source: ITRC 1,4-Dioxane Team, 2020.
In approaching a site with suspected 1,4-dioxane impacts, an investigator should evaluate whether sampling should be performed and what media should be sampled. This section will provide you with information to consider when making these determinations relative to 1,4-dioxane. Consider the following factors when deciding whether sampling should be performed, what media should be sampled, and where and how many samples should be collected:
- The regulatory environment (see Section 2)
- Site history (see Section 1)
- Currently available data and the established CSM (if available)
- Potential exposure risks (see Section 5)
- Site goals
- Potential exposure risks (see Section 5)
3.2.1 Media
Table 3-2 lists the media potentially impacted by 1,4-dioxane releases, along with information regarding why each medium may or may not be a concern given 1,4-dioxane’s physical and chemical properties. The table also provides an evaluation of whether each medium should be a priority for evaluating 1,4-dioxane and whether omitting that medium from assessment would have major or minor consequences or effect on conclusions reached for a given site. Finally, it proposes data to collect when the media is identified for evaluation. An idea of ranges of concentrations of 1,4-dioxane that have been reported in literature for each media can be found in Section 1.4.
Table 3-2. Considerations for evaluating media potentially impacted by 1,4-dioxane
| Medium | Key concerns | Importance to CSM | Data to collect when media is addressed |
| Groundwater | Partitioning and Transport: 1,4-Dioxane has high aqueous solubility and low sorption to soil, so it is expected to partition to and transport with water. (See Section 3.1 for additional discussion of processes affecting 1,4-dioxane movement in groundwater.)
Human Health and the Environment: Potential risk to users of impacted groundwater as a drinking water supply or as a source of contamination to surface water. (See Section 5.) |
High Consistent with its physicochemical properties, 1,4-dioxane has been identified as a widespread groundwater contaminant (see Section 1). |
• 1,4-Dioxane concentration • Concentration of colocated compounds of interest • Groundwater flow rate • Groundwater flow direction • Potential for matrix diffusion effects • Groundwater geochemistry • Groundwater use |
| Surface Water | Partitioning and Transport: 1,4-Dioxane is anticipated to persist within surface water due to its low Henry’s law constant, high aqueous solubility, and low potential for hydrolysis and photodegradation in water. (See Section 3.1.5) Human Health and the Environment: Because of 1,4-dioxane’s persistence within surface water bodies, and the generally low drinking water guidance values (typically single ppb values; see Figure 2-1), this risk is not necessarily limited to the areas proximate to the point or nonpoint discharge. |
High Potential groundwater to surface water connections and/or point sources to surface water should be evaluated. |
• Aqueous 1,4-dioxane concentrations • Concentration of colocated compounds of interest • Surface water use |
| Soil/Sediment | Partitioning and Transport: 1,4-Dioxane is not expected to persist in soil or sediment environments, and once released to this medium, it is expected to move rapidly with little retardation from sorptive mechanisms. (See Section 3.1.2 for additional discussion.) Soil may be an important media for a release of 1,4-dioxane under conditions where infiltration and/or leaching are minimal (e.g., soil under a building) or when the release is recent.
Human Health and the Environment: Potential for exposure when in contact with recently contaminated soils or soils protected from leaching. |
Medium Evaluation of soils and sediment should be based on the conditions of the release and the site history. |
Concentration of 1,4-dioxane in soil in areas of suspected release. |
| Indoor/Outdoor Air | Partitioning and Transport: In the absence of a pure phase* release to dry soil, or background sources (e.g., consumer products such as paints, adhesives, detergents, cleaning products, and personal care product Human Health and the Environment: 1,4-Dioxane is not typically considered a target compound for vapor intrusion concerns when dissolved in water but may pose a vapor intrusion concern if present in pure phase* or in the instance of groundwater intrusion into a building or use of contaminated groundwater. (See Section 5.1.2.) |
Low Indoor/outdoor air exposures are expected to be low except for the conditions noted. |
Concentration of 1,4-dioxane in air in areas of suspected release or where contaminated groundwater is used. |
| Aquatic Biota | Partitioning and Transport: 1,4-Dioxane has a low bioaccumulation potential (USEPA 2017d) and therefore is not a concern for biomagnification. Because of the low potential for adsorption to the sediments, ecological exposures to benthic organisms are expected to be low.
Human Health and the Environment: 1,4-Dioxane is not highly toxic to aquatic organisms at µg/L concentrations typically found in surface water [(Mohr et al. 2020); USEPA 2017d)]. Available aquatic bioassay results [(BUA 1991), as cited in (Mohr et al. 2020); (NICNAS 1998); (EC 2010); (USEPA 2015b); (USEPA 2018e)] show that 1,4-dioxane is acutely toxic to bacteria, algae, invertebrates, and fish at higher concentrations, ranging from hundreds to thousands of mg/L. Discussion of toxicity to aquatic receptors is available in Section 5.3.2.1. |
Low Ecological exposures for this medium are likely to be very low except for high-concentration sources. |
Develop biota sampling strategy based on concentration of 1,4-dioxane in water. |
| Terrestrial Biota | Partitioning and Transport: 1,4-Dioxane has a low bioaccumulation potential (USEPA 2017d) and therefore is not a concern for biomagnification. Human Health and the Environment: Little data is available on the effect of direct exposure to 1,4-dioxane on terrestrial organisms (USEPA 2018b). Exposures to terrestrial wildlife through the food chain are not significant with respect to the levels of 1,4-dioxane typically observed in contaminated soils [(Huntley, Amarai, and Schell 2004), as cited in (Mohr et al. 2020)]. Further discussion of toxicity to terrestrial receptors is available in Section 5.3.2.2. |
Low Ecological exposures for this medium are likely to be very low except for recent spills and pure phase* spills. |
Tissue concentrations of 1,4-dioxane in organisms of concern if warranted by site-specific conditions. |
*Pure phase is technical grade (i.e., >95% 1,4-dioxane) or American Chemical Society (ACS) reagent grade (i.e., >99% 1,4-dioxane) product.
In addition to knowing the general concerns regarding 1,4-dioxane in a medium and its relative importance in the assessment process, the investigator should also consider site history and usage before determining the investigative path forward. There are several questions related to site operations, site history, and chlorinated solvent use that can help the investigator evaluate the likelihood of 1,4-dioxane being present and determine media to be sampled (DiGuiseppi and Mahler 2015). 1,4-Dioxane is expected to co-occur with chlorinated solvents based on its use as a stabilizer for 1,1,1-TCA and potentially TCE (see Section 1). Consider any other known circumstances or site activities that may have included 1,4-dioxane handling or use before reaching any conclusions on sampling for 1,4-dioxane. Potential uses and sources of 1,4-dioxane are discussed in Section 1.
Figure 3-4 presents a visual set of guidelines to help readers with the thought processes required in considering what, if any, 1,4-dioxane characterization activities may be required at a given site, and to help focus the rationale for sampling and media to be sampled. In this flow chart, ITRC attempted to capture the most common circumstances for sites potentially impacted with 1,4-dioxane. Clearly, not all possible site circumstances can be captured in such an abbreviated effort, and this guidance may not be applicable to certain sites. Consider this flow chart as general guidance, and take site-specific aspects into consideration before planning the investigation. Specifically, reviewing the CSM and potentially revising it in the context of 1,4-dioxane occurrence, release, or migration is advised. Nothing in the flow chart should be interpreted to be absolute; instead, the chart should serve as a catalyst for discussions among site stakeholders.
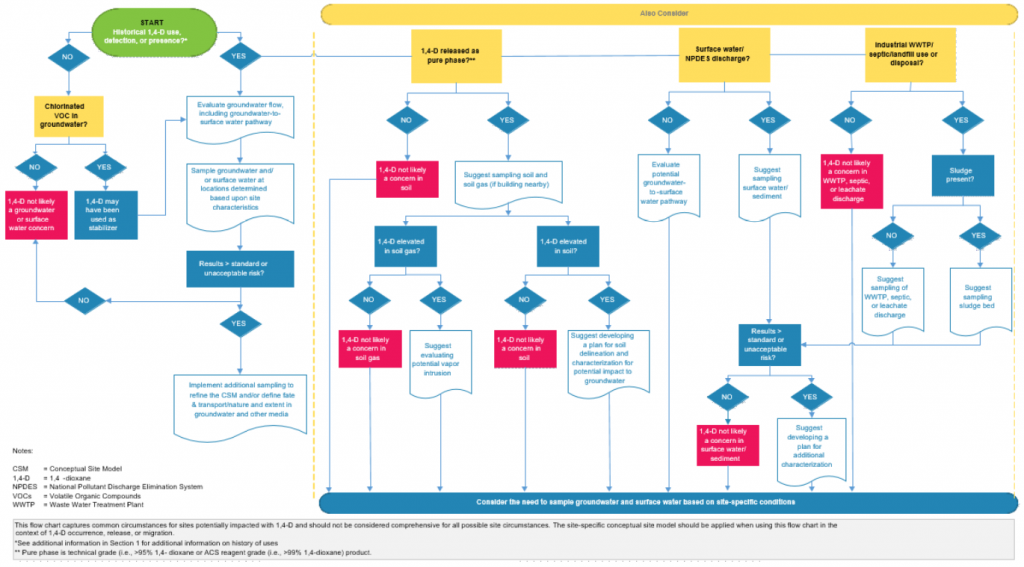
Figure 3-4. General screening tools for initial 1,4-dioxane assessment
Source: ITRC 1,4-Dioxane Team, 2020.
Notes: Figure 3-4 presents a graphic that can be used to guide assessment activities for potential 1,4-dioxane sites. Please note that this should not be considered comprehensive or definitive. Site-specific factors will affect the CSM, and this figure should be used in conjunction with a thorough assessment of sources, pathways, and receptors by knowledgeable professionals.
3.2.2 Groundwater Investigation Considerations
As described in the preceding sections, groundwater is likely to be the dominant media of concern for 1,4-dioxane investigations. Investigation of the distribution of 1,4-dioxane in the environment should be guided by an understanding of the site geology, hydrogeology, media-specific flow properties, geochemistry, and locations and characteristics of potential sensitive receptors. These characteristics are used to develop a CSM that can be relied on for decision-making and updated throughout the investigation. A successful groundwater investigation generally has the following goals:
- To understand dominant contaminant source areas and mass flux from these areas
- To understand the extent of contamination, as defined by delineation to relevant regulatory criteria
- To understand plume dynamics, including whether the plume is stable, shrinking, or expanding
While most 1,4-dioxane soil source areas are expected to be short-lived, matrix back-diffusion has the potential to contribute to long-term plume persistence. As a result, it may require more effort to identify a “high-value” target for aggressive source treatment, and in fact it may be difficult to identify the source itself at some sites. Successful site investigation strategies are designed to collect 1,4-dioxane concentration data and lithologic data needed to characterize where 1,4-dioxane mass has concentrated (e.g., in fine-grained intervals) and to estimate the rate and magnitude of the ongoing mass flux potential from these locations. Understanding of site stratigraphy (and thereby potential groundwater storage and transport zones) is critical in assessing the presence and prevalence of 1,4-dioxane throughout groundwater plumes. High-resolution site characterization and other methods for investigating site-specific geology contribute to this understanding.
As described previously, 1,4-dioxane has the potential to travel at the approximate velocity of groundwater, and this characteristic is particularly important in high-permeability zones (e.g., in coarser-grained intervals) where transport rates are significant. As a result, 1,4-dioxane groundwater investigations should be targeted to understand the distribution of 1,4-dioxane in the context of aquifer flow parameters. This may be achieved by using a simple or a complex fate and transport model to predict the extent of an uncharacterized 1,4-dioxane plume, and then using the predicted extent to select confirmatory sampling locations. An example of a simple fate and transport model is one-dimensional steady state with single degradation rate constant, while a more complex model may be three-dimensional with consideration of matrix diffusion and degradation characteristics over space and time. Results from sampling locations selected to achieve plume delineation may also be used to refine understanding of fate and transport processes.
Groundwater monitoring over space and time is critical to understanding plume dynamics. Thus, a successful groundwater investigation strategy will establish monitoring points that allow for ongoing plume monitoring and characterization. A change in the extent of a groundwater plume and/or a trend in concentrations over time may indicate either an increasing or a decreasing plume extent. In the case of an increasing plume extent, unidentified 1,4-dioxane sources may be present or attenuation processes (including destruction via biodegradation) may not be effective. Conversely, in the case of a decreasing plume extent, a line of evidence for effective attenuation may be established and supported by advanced characterization methods for 1,4-dioxane attenuation (see the Remediation Fact Sheet). While not all groundwater models have a strong predictive capacity, some models may provide important insight into plume characteristics and may provide a supporting line of evidence for characterizing plume dynamics. While there is empirical evidence that 1,4-dioxane’s footprint in groundwater at many sites may not necessarily be distinct from that of co-occurring CVOCs like 1,1-DCE (Adamson et al. 2014a), the potential for rapid migration and relatively low regulatory standards in some jurisdictions are key considerations and may lead to additional delineation requirements specifically for 1,4-dioxane. Two key factors that may influence the distribution of 1,4-dioxane relative to co-occurring CVOCs are the relative duration of release of 1,4-dioxane versus CVOCs, and the amount of 1,4-dioxane included in solvent mixtures. Chlorinated solvents were in wide industrial use approximately 20 years before 1,4-dioxane was added as a stabilizer to 1,1,1-TCA. As such, the duration of a 1,4-dioxane release may be less than the duration of a CVOC release, leaving 1,4-dioxane contamination less time to travel in groundwater from the source (see Figure 3-1). Additionally, the amount of 1,4-dioxane released as a result of its use as a stabilizer for CVOCs would likely have been less than the amount of chlorinated solvent released in the waste product (see Section1). As such, although 1,4-dioxane may be more mobile than co-occurring CVOCs, the overall plume footprint may be smaller. However, as discussed above, this should be evaluated on a site-by-site basis.