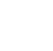6. Remediation and Treatment Technologies
The concepts in Section 3, Section 4, and Section 5 help indicate whether 1,4-dioxane remediation or treatment is needed. This section provides details on the most commonly used treatment technologies as well as information on popular technologies that aren’t the best choice for 1,4-dioxane. Specifically, this section provides information on the characteristics that affect treatment selection, drinking water treatment options, wastewater treatment options, residential water treatment options, soil treatment options, and both in situ and ex situ groundwater treatment options.
This section is not meant to serve as a stand-alone resource for choosing a treatment technology. A given site’s specifics (i.e., the hydrogeological CSM, the potential risk-pathway CSM, and the regulatory framework) must be considered during the technology selection process. The 1,4-Dioxane Remediation and Treatment Technologies Fact Sheet provides a high-level overview of ex situ and in situ treatment technologies. Of particular interest, Tables 1 and 2 of the fact sheet provide a screening-level tool for identifying 1,4-dioxane treatment technologies. This document offers readers more details related to various design and implementation considerations associated with each treatment technology. In both the fact sheet and this document, technologies are categorized as “fully demonstrated,” “emerging option,” or “less effective.” Fully demonstrated technologies are those that have been implemented or demonstrated under full-scale situations. These typically include effective, well-documented treatment technologies. Emerging options may be partially demonstrated or researched and may include technologies that have been implemented at the bench- and/or pilot-scale. Less effective technologies are those with negligible or limited capacity for 1,4-dioxane removal, either by demonstration or theoretical considerations. Over time the categories presented herein may become outdated, particularly as “Emerging Options” become “Fully Demonstrated.”
Figure 6-1 was developed to aid the reader in navigating these various remediation and treatment technologies. Hovering over the various icons in the web-based version of this document will bring up the relevant remediation and treatment technologies for that environmental matrix, categorization, or level of contamination. Clicking on the desired treatment technology will navigate the reader to that portion of the document. Once readers finish reviewing that section, they can navigate back to Figure 6-1 to explore other technologies. Lastly, there are case studies that have been compiled that illustrate pilot-test and full-scale demonstrations of several of the treatment technologies available.
XSVE
Traditional Soil Technologies
-Excavation (Fully Demonstrated) (Section 6.3.1.1)
-Thermal Desorption (Fully Demonstrated) (Section 6.3.1.2)
-Solidification/Stabilization (Fully Demonstrated) (Section 6.3.1.3)
-Oxidant Soil Blending (Emerging Option) (Section 6.3.2.2)
-Conventional SVE (Less Effective) (Section 6.3.3.1)
-Bioventing/Bio-Piles (Less Effective) (Section 6.3.3.2)
Thermal
In-Situ Chemical Oxidation
Metabolic Bioremediation
Phytoremediation
Pump and Treat with Ex-Situ Treatment
- AOPs (Fully Demonstrated) (Section 6.2.4.1)
- Aerobic Bioreactor (Emerging Option) (Section 6.4.2.1)
- Sorptive Resin (Fully Demonstrated) (Section 6.4.1.2)
Monitored Natural Attenuation
Electrochemical
Cometabolic Bioremediation
Ex-Situ Treatment
- AOPs (Fully Demonstrated) (Section 6.2.4.1)
- Aerobic Bioreactor (Emerging Option) (Section 6.4.2.1)
- Sorptive Resin (Fully Demonstrated) (Section 6.4.1.2)
Wastewater Treatment
- Conventional Wastewater Treatment (Section 6.2.2)
- Industrial Wastewater Treatment Options (Section 6.2.3)
- Advanced Wastewater Treatment Options (Section 6.2.4)
Drinking Water Treatment
- Conventional Drinking Water Treatment (Section 6.2.1)
- Advanced Drinking Water Treatment Options (Section 6.2.4)
Residential Drinking Water Treatment
Figure 6-1. Remediation and treatment technologies.
Source: ITRC 1,4-Dioxane Team, 2020.
6.1 Characteristics That Affect Treatment Selection
1,4-Dioxane is a heterocyclic compound that is fully miscible in water. In aqueous solution, 1,4-dioxane is chemically stable and does not undergo abiotic hydrolysis; however, in aqueous solution, it is susceptible to chemical oxidation by strong oxidizing agents (e.g., hydroxyl radicals; see Section 6.5.1.1) and aerobic (but not anaerobic) biological oxidation (see Sections 6.5.2.2 and 6.5.2.3). At standard conditions, the vapor pressure of pure 1,4-dioxane at 25°C is 38.1 mm Hg.
Its high aqueous solubility results in a low Henry’s law constant of 4.80 × 10−6 atm m3/mol. Consequently, some conventional remediation approaches, such as air stripping and soil vapor extraction (SVE), are largely ineffective for the remediation of 1,4-dioxane contamination. Exceptions would be if the volatility of dissolved 1,4-dioxane is increased through the use of heated matrix (see Section 6.3.2.1 for an example) or if certain site-specific conditions (e.g., arid climate, separate phase 1,4-dioxane) are present.
1,4-Dioxane also has low n-octanol-water (Log Kow of -0.27 to -0.42) and carbon-water (Log Koc 0.42 to 1.46) partitioning coefficients. These characteristics, in conjunction with the source of 1,4-dioxane, affect the type of groundwater plume that is formed. For example, release of 1,4-dioxane as a solvent stabilizer can result in the formation of dilute plume (e.g., <100 µg/L), whereas release of pure 1,4-dioxane can result in a plume with much higher concentrations (e.g., >1,000 µg/L). These characteristics strongly influence the type of in situ aerobic biodegradation processes (e.g., cometabolism vs. metabolism) that either can be engineered or can contribute to the natural attenuation of 1,4-dioxane.
The efficacy of these biological treatments is also further influenced by the potential presence of chlorinated co-contaminants such as 1,1,1-TCA, 1,1-DCE, TCE, and cis-1,2-dichloroethene (cis-1,2-DCE) (Zhang, Gedalanga, and Mahendra 2016). The low partitioning coefficients (Kow and Koc) also considerably limit the efficacy of common sorbents such as granular activated carbon (GAC) as remediation approaches for 1,4-dioxane, although specialized sorbents can be used to remove dissolved 1,4-dioxane from contaminated water (see Section 6.4.1.2).
6.2 Drinking Water and Wastewater Treatment
1,4-Dioxane treatment in drinking water is required in states where drinking water standards for 1,4-dioxane are established. Occasionally, removal of 1,4-dioxane is required in wastewater, particularly industrial wastewater. This section discusses 1,4-dioxane removal by treatment processes commonly used in the water and wastewater industry.
Readers should recognize that the concentrations of 1,4-dioxane in various matrices can vary by orders of magnitude. The difference in 1,4-dioxane concentrations and co-contaminants, target cleanup levels, and the different water quality matrices (e.g., a relatively “clean” groundwater as source for drinking water vs. an industrial wastewater containing complex matrix and high concentrations of total organics) must be considered when evaluating each treatment technology.
In general, 1,4-dioxane is challenging to remove from drinking water due to its physical and chemical properties. Many conventional unit processes involved with drinking water treatment are ineffective in 1,4-dioxane treatment. Therefore, a water treatment plant (WTP) is not expected to remove 1,4-dioxane unless it has one of the following treatment processes:
- Advanced oxidation processes (AOPs) (Section 6.2.4.1)
- Ozone (under certain conditions) (Section 6.2.4.2)
- Reverse osmosis (RO), at various efficiencies (Section 6.2.4.3)
AOPs are a group of technologies that use the highly reactive hydroxyl radical to destructively remove organic contaminants and are the only fully demonstrated technologies available for 1,4-dioxane treatment in drinking water and groundwater (Section 6.4.1.1). Additionally, two more water treatment processes, ozone (under some conditions), and RO were found to remove 1,4-dioxane at various efficiencies in laboratory studies and full-scale plants. If a WTP has an ozone or RO unit process, it is possible that 1,4-dioxane concentrations may be reduced in the treatment process.
1,4-Dioxane can also be removed by GAC, but the breakthrough occurs much faster than more hydrophobic VOCs. Therefore, it is possible to use GAC to treat 1,4-dioxane at low flow rates, including as a point-of-entry treatment (POET) approach, but the GAC’s adsorption capacity is expected to be exhausted quickly if the flow rate is high. As with adsorption of other contaminants, the effectiveness of 1,4-dioxane adsorption can also be affected by the water quality matrix.
1,4-Dioxane is not effectively treated by conventional unit processes involved with municipal WWTPs. In theory, treating 1,4-dioxane in wastewater by AOPs is achievable, but in practice, the efficiency of such systems depends on the quality of the wastewater and should be evaluated on a case-by-case basis. In industrial wastewater, such as plastic manufacturing wastewater and landfill leachate, where both the bulk organic and 1,4-dioxane concentrations are relatively high, biological treatment has emerged as a method to remove a substantial amount of 1,4-dioxane. However, despite high removal efficiencies and potentially being able to meet the wastewater discharge limits, the treated 1,4-dioxane concentration is likely to remain higher than the regulatory standards, unless additional treatment occurs afterward.
6.2.1 Conventional Drinking Water Treatment
The historical focus of drinking water treatment is to remove waterborne bacteria, acutely toxic contaminants, and aesthetic nuisances. Screening, coagulation, sedimentation, granular filtration, and disinfection and oxidation are typical conventional drinking water treatment processes when surface water is used as the source water (Crittenden et al. 2012). Aeration and adsorption are additional treatment processes for groundwater and surface water that have been used for the treatment of VOCs.
1,4-Dioxane’s physical and chemical properties suggest that these treatment processes are not expected to remove 1,4-dioxane. For this reason, few studies have investigated the treatment of 1,4-dioxane by conventional water treatment processes [(McGuire, Suffet, and Radziul 1978); (DiGuiseppi and Whitesides 2007)]. McGuire et al. (McGuire, Suffet, and Radziul 1978) evaluated processes including coagulation, aeration, chlorination, permanganate oxidation, GAC, and powdered activated carbon (Smith et al.) for 1,4-dioxane treatment. Except for GAC, none of the processes evaluated by McGuire et al. (McGuire, Suffet, and Radziul 1978) were able to remove 1,4-dioxane. GAC removed 67% of the 1,4-dioxane after 65 bed volumes (BVs) at a relatively short empty bed contact time (EBCT) (1.1 to 2.1 minutes) and relatively high initial concentrations (on the order of 1 mg/L). DiGuiseppi and Whitesides (DiGuiseppi and Whitesides 2007) tested the treatment of 1,4-dioxane by an air stripping (aeration) tower at a groundwater remediation site. The maximum possible removal rate achieved was 10% using extreme air/water ratios between 183 and 291, which is not practical in water treatment.
These results are supported by recent WTP sampling studies. Stepien et al. (Stepien et al. 2014) sampled two WTPs using surface water as the source water and found no observable removal of 1,4-dioxane. Knappe et al. (Knappe et al. 2016) came to a similar conclusion for two of the three surface water treatment plants sampled in their study. These four plants with little 1,4-dioxane removal included a range of treatment processes, including riverbank filtration, ozonation, aeration, GAC filtration, sand/gravel filtration, coagulation, flocculation, sedimentation, chlorination, chloramination, and PAC adsorption. Knappe et al. (Knappe et al. 2016) observed an average of 67% 1,4-dioxane removal in a third WTP, which the authors attributed to the two ozonation processes in the presence of natural organic matter (NOM)—that is, the background organics present in waters (higher in surface water than in groundwater). This will be further discussed in Section 6.2.4.2.
6.2.2 Conventional Wastewater Treatment
The removal efficiencies for 1,4-dioxane under typical wastewater treatment conditions are expected to be very low based on the available information on biodegradation, sorption, and air stripping. Abe (Abe 1999) examined 1,4-dioxane concentrations in the effluent from three large wastewater treatment systems (18, 53, and 79 million gallons per day [MGD]) in Japan receiving both municipal and industrial wastes. The effluent concentration ranges for 1,4-dioxane at these plants were 1.0–88, 3.6–97, and 1.7–3.0 μg/L. The 1,4-dioxane removal efficiency for the 53 MGD plant varied from 0%–31% during two sampling events. The removal efficiencies for the other two plants were not reported.
Sampling of three WWTPs in Germany also reported negligible 1,4-dioxane removal (Stepien et al. 2014). Interestingly, the effluent of a fourth WWTP was found to contain significantly higher concentrations of 1,4-dioxane than the influent. The authors identified the source of added 1,4-dioxane as the methanol used to provide the organic carbon for the denitrification process, which contained up to 2.2 mg/L of 1,4-dioxane (Stepien et al. 2014).
In the United States, a total of 40 municipal WWTPs that receive predominantly domestic wastewater from households were monitored for 1,4-dioxane in 2010 (Simonich et al. 2013). The influent 1,4-dioxane concentrations were not reported, but the effluent concentrations ranged from below the detection limit (<0.3 μg/L) to 3.3 μg/L, with a mean of 1.13 μg/L.
6.2.3 Aerobic Biological Treatment for Industrial Wastewater
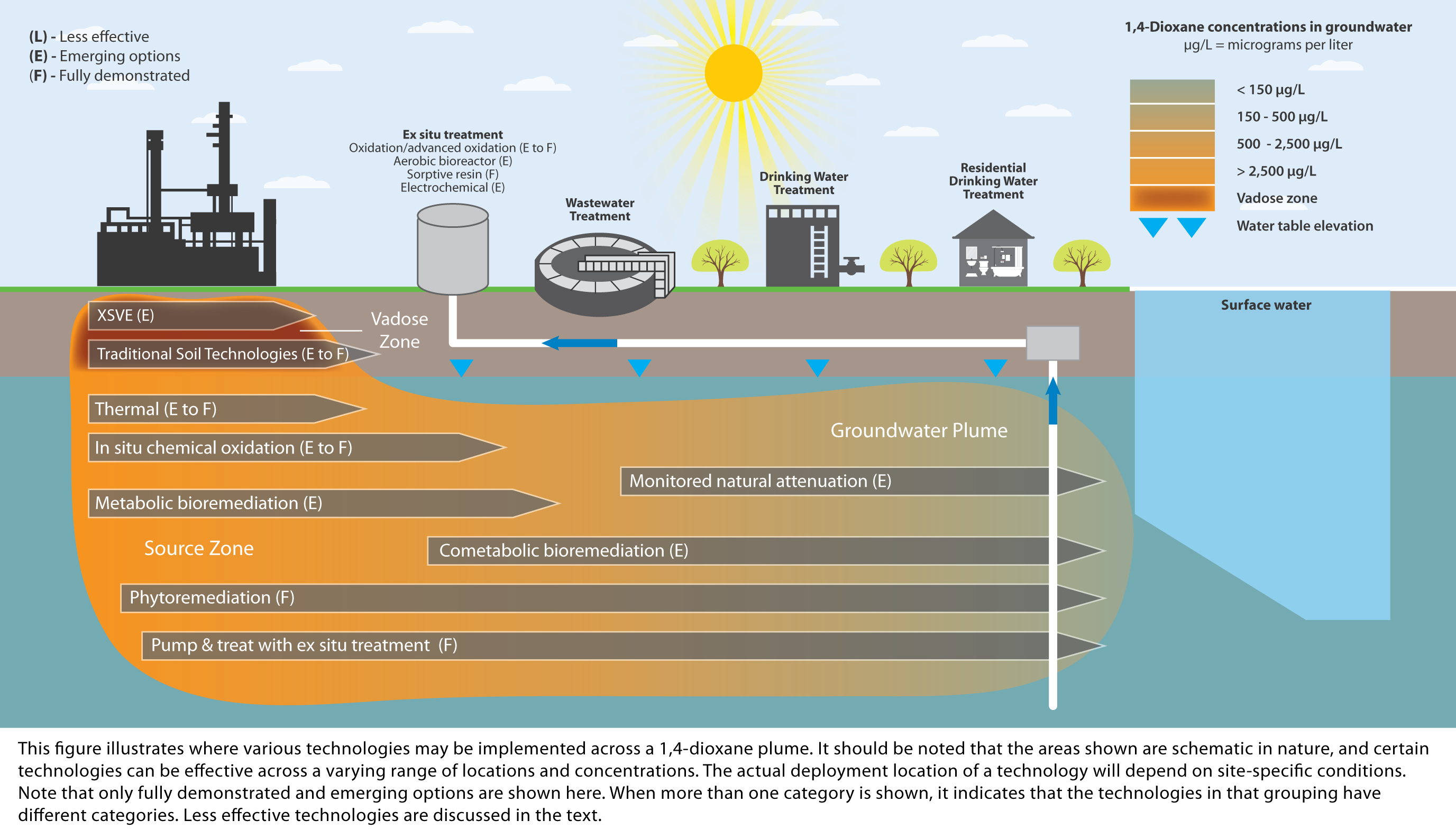
Microorganisms can either use 1,4-dioxane as a sole carbon and energy source for growth or degrade 1,4-dioxane concomitantly when growing on another carbon and energy source. The first form is generally referred to as “metabolic degradation” and the latter as “cometabolic degradation.” The chemicals that can be used as a carbon and energy source by bacteria performing 1,4-dioxane cometabolism include THF, short-chain alkanes (which include methane, ethane, propane, butane, and isobutane), and toluene.
It is generally believed that metabolic degradation is better suited for treating higher concentrations of 1,4-dioxane (such as those encountered in industrial wastewaters) ((Barajas-Rodriguez and Freedman 2018); (McElroy and Hyman 2019)). Cometabolic degradation has the potential to drive the 1,4-dioxane concentration to low μg/L levels but will require additional equipment to handle and efficiently deliver the primary growth substrate(s) which are often potential fire and explosion hazards, unless they are present in the liquid stream being treated.
Some industrial wastewater contains 1,4-dioxane with concentrations at 10 to several hundred mg/L. When 1,4-dioxane reduction is required, aerobic biological treatment can be an effective treatment option. In fact, many early 1,4-dioxane biodegradation studies were performed for industrial wastewater treatment [(Sandy et al. 2001); (Sock 1993); (Zenker, Borden, and Barlaz 2004)]. These studies found 1,4-dioxane can be degraded quite efficiently by mixed cultures enriched from bioreactors treating complex industrial wastewaters in the plastics manufacturing industry. Some of the cultures were able to metabolize 1,4-dioxane [(Sandy et al. 2001); (Sock 1993)], and some of them degraded 1,4-dioxane only in the presence of THF (Zenker et al. 2004). More recently, more microbial cultures, including pure isolates, were found to be capable of 1,4-dioxane degradation. Biodegradation of 1,4-dioxane is further reviewed in Sections 6.5.1 and 6.5.2.
Like other bioreactors used in industrial wastewater treatment, biological treatment of 1,4-dioxane can be achieved in common types of suspended-growth or attached-growth (i.e., biofilm) reactors. Depending on the type of bioreactors, required reaction conditions, and influent water quality, a biological treatment system may comprise the following:
• A reactor vessel
• Equipment to provide mixing (if required)
• Aeration systems (if additional oxygen is required)
• Nutrient injection systems (for nitrogen and phosphorous, if required—and, rarely, micronutrients such as trace metals)
• Equipment for biomass/liquid separation and recycle (if required)
• Other supplemental systems (e.g., for pH control)
The following sections provide an overview of the existing body of literature on biological 1,4-dioxane treatment in industrial wastewater.
6.2.3.1 Metabolic 1,4-Dioxane Treatment by Bioreactors in Industrial Wastewater
(Sock 1993) operated an aerobic fluidized bed reactor with a mixed microbial culture capable of using 1,4-dioxane as the sole carbon and energy source. After the initial startup, 100 mg/L of 1,4-dioxane was removed to below 1 mg/L for over 1 month.
(Sandy et al. 2001) pilot tested various combinations of anaerobic bioreactors, aerobic bioreactors, distillation, and AOP technologies for treatment of industrial wastewater. The aerobic bioreactors were seeded with the activated sludge from the existing activated sludge treatment system operating at the industrial facility as well as a 1,4-dioxane-degrading enrichment culture obtained from Clemson University.
One configuration consisting of biological treatment trains (anaerobic/aerobic/aerobic) achieved effluent concentrations consistently below 40 μg/L for over 30 days. In that configuration, the anaerobic bioreactor did not appreciably remove 1,4-dioxane as expected. The first aerobic stage removed 1,4-dioxane from an average of 430 mg/L to 0.5 mg/L. Waste sludge from the first aerobic reactor provided continual seeding of 1,4-dioxane degrading microorganisms to the second aerobic stage. The reduction of 1,4-dioxane in the second-stage aerobic bioreactor to the final average concentration of 40 μg/L may be an effect of both dilution and destructive treatment via biodegradation, since a dilute wastewater stream was mixed with the effluent of the first aerobic stage and the combined stream was the influent to the second-stage aerobic bioreactor.
6.2.3.2 Cometabolic 1,4-Dioxane Treatment by Bioreactors in Industrial Wastewater
(Zenker, Borden, and Barlaz 2004) evaluated cometabolic 1,4-dioxane removal in the presence of THF in a trickling bioreactor at the bench scale. The influent 1,4-dioxane concentrations ranged from 0.2 to 1.25 mg/L, and the influent THF concentrations were between 6 and 22 mg/L. The 1,4-dioxane removal efficiency throughout the 1-year bench test was 93%–97%. The lowest effluent 1,4-dioxane concentration of 9 μg/L was achieved when the THF concentration was high (22 mg/L) and the 1,4-dioxane influent concentration was low (0.2 mg/L).
6.2.3.3 Full-Scale Cometabolic 1,4-Dioxane Treatment by Bioreactors in Landfill Leachate
A full-scale moving-bed bioreactor (MBBR) system was installed at the Lowry Landfill Superfund site (Lowry) in Colorado to treat 1,4-dioxane in 2004 (Cordone et al. 2016). MBBR is an attached-growth bioreactor in which the plastic biofilm carriers are suspended and well-mixed with the liquid. Immediately after startup, the MBBR was operated at a relatively high 1,4-dioxane concentration of 15,000 to 25,000 μg/L and a long hydraulic retention time (HRT) of approximately 1.5 days. The average 1,4-dioxane removal was greater than 95% during this time.
Over time, the MBBR operations departed from the initial design. In the 12 months between July 2015 and June 2016, the system was treating a much lower influent 1,4-dioxane concentration of generally 1,000 to 2,000 μg/L (1,326 μg/L on average) at a much shorter HRT of approximately 12 hours. The reactor performance generally was not affected by these conditions, achieving on average a 1,4-dioxane removal efficiency of 92% and an effluent concentration of 93 μg/L. Regarding the possible degradation mechanism, the authors speculated that it was probably cometabolic, with THF concentrations typically at double or triple the concentrations of 1,4-dioxane throughout its implementation history (Cordone et al. 2016).
6.2.4 Advanced Treatment Processes for Drinking Water and Wastewater
Over time, more advanced treatment processes were developed and implemented to treat trace amounts of contaminants, or to produce drinking water from more challenging source waters (e.g., seawater or brackish groundwater). In general, these more robust advanced treatment technologies are also more costly to implement. Example of advanced water treatment technologies include AOPs, ion exchange (Félix-Navarro et al. 2007), and membrane filtration, which includes microfiltration (Kambhu et al. 2017), ultrafiltration (McGuire, Suffet, and Radziul 1978) nanofiltration (NF), and RO.
6.2.4.1 Advanced Oxidation Processes
Conventional oxidants used in water treatment, including chlorine, chloramine, chlorine dioxide, potassium permanganate, and molecular ozone, are generally ineffective in treating 1,4-dioxane under conditions relevant to drinking water and wastewater treatment (ozone is an exception under certain conditions; see Section 6.2.4.2). AOPs were developed to provide an even stronger oxidant, the hydroxyl radical, for the oxidation of recalcitrant organic contaminants (Crittenden et al. 2012). While other oxidants can be generated under AOPs, the hydroxyl radical is one of the strongest oxidizing agents available and can completely mineralize 1,4-dioxane [e.g., (Adams, Scanlan, and Secrist 1994.); (Otto and Nagaraja 2007); (Stefan and Bolton 1998)].
Due to its high oxidation power, hydroxyl radical reacts with organic contaminants rapidly and indiscriminately. This is evidenced by the large second-order reaction rate constants between the hydroxyl radical and many contaminants [e.g., (Crittenden et al. 2012)]. The competition for hydroxyl radicals with other contaminants and general water quality parameters becomes important when large quantities of competing compounds are present in the water to be treated.
AOPs are by far the most common technology for 1,4-dioxane treatment and remain to be the only available category of technologies for drinking water treatment. A case study highlighting the use of AOPs for drinking water treatment is included here. AOPs are differentiated based on the process to generate hydroxyl radicals. Processes that are known to generate hydroxyl radicals include the combination of the following:
- UV light and hydrogen peroxide (UV/H2O2)
- Ozone and hydrogen peroxide (O3/H2O2)
- UV light and titanium dioxide (UV/TiO2)
- UV light and chlorine (or hypochlorite) (UV/Cl2)
Other hydroxyl radical-generation processes (e.g., persulfate/UV, ozone/ultrasound, nonthermal plasma) exist and can also treat 1,4-dioxane [(Dietrich et al. 2017); (Even-Ezra et al. 2009); (Li et al. 2017); (Li et al. 2018); (Xiong et al. 2019)]. They are not discussed further because there are no commercially available treatment systems for these AOPs. A case study for 1,4-dioxane treatment by UV/H2O2 is included here.
Water quality parameters that compete for hydroxyl radicals (e.g., background organics, carbonate/bicarbonate ions, chloride) can significantly impact treatment efficiency for all AOP types.
For the UV/H2O2 process, other disadvantages include high energy use, periodic replacement for UV lamps, and water quality parameters that affect the transmission of the UV light (e.g., suspended solids, background organics, and nitrate). Additional processes may be required to remove the residual H2O2 by catalytic GAC or chemicals.
For the O3/H2O2 process, other disadvantages include the formation of bromate (from the oxidation of bromide ion) and high chemical use. Bromate is a regulated drinking water contaminant with an MCL of 10 μg/L. Optimized reactor design can reduce bromate formation. As with the UV/H2O2 process, H2O2 quenching may be needed.
The UV/TiO2 process requires UV photons at a lower energy (i.e., longer wavelength) compared to that required for the activation of H2O2. Thus, it improves energy consumption and UV transmission. However, fouling of the TiO2 catalyst may occur, and a mechanical process is needed to separate the TiO2 from the treated water prior to discharge.
The UV/Cl2 process has recently become an alternative to the UV/H2O2 process. It overcomes some disadvantages of the UV/H2O2 AOP but can only operate cost-effectively if the pH in the feed water is low (e.g., <6).
A unique aspect of implementing an AOP process is that the impact of water quality must be carefully considered. Specifically, the following compounds react with hydroxyl radicals at sufficiently fast rates that can significantly interfere with the treatment of the target contaminant:
- Carbonate species (carbonate and bicarbonate; particularly at higher pH)
- Chloride and bromide
- Background organics
- Reduced metal ions (iron and manganese)
For most groundwater, the AOP efficiency is only mildly affected by inorganic interferences, and the level of background organics (i.e., NOM) is low. The main notable exception is if nitrate is present in the groundwater at high concentrations, which can absorb significant UV light; however, for surface water and wastewater, the background organics, which directly compete for hydroxyl radicals and have strong UV absorbance, can greatly increase the chemical and energy consumption and may be detrimental for the removal of the target contaminant [(Crittenden et al. 2012); (Knappe et al. 2016); (Otto and Nagaraja 2007)]. Treatability studies are recommended for all types of waters, particularly for surface water and wastewater.
Another practical consideration for 1,4-dioxane treatment by AOPs is the removal of co-contaminants. Although the hydroxyl radical is a strong oxidant, the reaction rate with some of 1,4-dioxane’s co-occurring contaminants, namely chlorinated ethanes (e.g., 1,1,1-TCA, 1,1-DCA, 1,2-dichloroethane [1,2-DCA]) is notably slower. Therefore, if chlorinated ethanes are present at high concentrations in the water, additional treatment processes may be needed. Generally, chlorinated ethenes (e.g., TCE and 1,1-DCE) are readily removed by AOPs.
Due to these system complexities and operational constraints, AOP systems for 1,4-dioxane treatment are expected to have high capital and operation and maintenance (O&M) costs. The high O&M demands (e.g., handling of chemicals, complex mechanical and electrical equipment) also require availability of highly skilled operators.
6.2.4.2 Ozonation
Although ozonation has not been previously recognized as a 1,4-dioxane treatment technology in water and wastewater, its application has the potential of 1,4-dioxane removal under conditions favorable for the generation of hydroxyl radical. As discussed below, increasing evidence indicates ozonation under some conditions can beneficially remove 1,4-dioxane.
Hydroxyl radicals are generated by ozone at pH >8 to 10 (Crittenden et al. 2012). This process has been explored for 1,4-dioxane removal (Andaluri and Suri 2017). pH adjustment is impractical for full-scale systems, and even at high pH, high ozone dosages were required to remove 1,4-dioxane. In addition, the removal was hindered in actual groundwater samples compared to deionized water (Andaluri and Suri 2017).
Additional conditions that favor the generation of hydroxyl radicals and the treatment of 1,4-dioxane include the following:
- Ozone with and without ultrasound in drinking water (Dietrich et al. 2017)
- Ozone in high-strength organic wastewater (Barndõk, Cortijo, et al. 2014)
- Ozone in treated WWTP effluent (Tackaert et al. 2019)
- Ozone in surface water (Knappe et al. 2016)
An interesting common theme is the role of organic matter in the promotion of the hydroxyl radical generation. In fact, the reaction of ozone with organic matter in surface water to produce hydroxyl radicals has long been recognized as the most important mechanism to destroy target compounds (Crittenden et al. 2012). Notably, >95% 1,4-dioxane removal was achieved when ozone was added to surface water samples from the Cape Fear River at 3.5 mg/L, or an ozone to total organic carbon (TOC) ratio of 1.25 (Knappe et al. 2016). Consistent with laboratory studies, it was found that a surface WTP in the Cape Fear River Basin practicing ozonation in two separate treatment steps removed 67% of 1,4-dioxane from the raw water (Knappe et al. 2016). A similar treatment mechanism involving ozone in oxidizing 1,4-dioxane in situ is described in Section 6.5.1.1.3.
Two reports of enhanced 1,4-dioxane treatment by ozone suggest the addition of GAC in the ozonation process may further enhance the formation of hydroxyl radical and removal efficiency in ultra-pure water (Tian et al. 2017) and aerobically treated nitrified domestic wastewater (Vatankhah et al. 2019). Nitrification prior to ozone treatment removed ammonia that would have reacted with, and consumed additional, ozone (Singer and Zilli 1975). Nearly half (i.e., 40%) of the 1,4-dioxane was removed from the aerobically treated nitrified domestic wastewater, which contained more than 5 mg/L of dissolved organic carbon (Vatankhah et al. 2019). The enhanced 1,4-dioxane removal may have resulted from the presence of both organic matter and GAC.
6.2.4.3 Nanofiltration and Reverse Osmosis
As with ozone, NF and RO are not recognized 1,4-dioxane treatment processes. 1,4-Dioxane’s low molecular weight and neutral charge do not favor separation by NF and RO. However, removal of 70%–90%, or even higher, of 1,4-dioxane has been reported under conditions relevant to water treatment [(Fujioka et al. 2018); (Kegel et al. 2010); (NHDES 2019)]. More limited information on 1,4-dioxane removal by NF is available, but one bench study showed that an NF90 membrane rejected approximately 40% of 1,4-dioxane (Yangali-Quintanilla et al. 2011).
Overall, existing data suggest that RO, and potentially NF, when installed for the treatment of other contaminants (e.g., total dissolved solids), can be considered a beneficial barrier for 1,4-dioxane, especially if the concentration in the raw water is very low. The removal efficiency is unlikely to be sufficient for higher initial 1,4-dioxane concentrations. Also, membrane filtration itself is expensive to implement, and the treatment is nondestructive.
6.2.4.4 Electrochemical Treatment
Electrochemical treatment (also known as advanced electrochemical oxidation or electrochemical AOP) is an emerging technology in which direct current is applied to drive the degradation of contaminants. The contaminant degradation occurs through reactive radicals (hydroxyl radicals, in particular) generated from the oxidation of water or via direct electron transfer at the electrode surface. Therefore, electrochemical oxidation could be considered another AOP; however, it is separately discussed here.
Bench tests have shown electrodes made from boron-doped diamond [(Barndõk, Hermosilla, et al. 2014); (De Clercq et al. 2010)] and catalytic metal oxide (Park, Mameda, and Choo 2018) are effective in oxidizing 1,4-dioxane in batch tests. Blotevogel et al. (Blotevogel et al. 2019) performed the first pilot test on 1,4-dioxane removal by electrochemical systems using mixed metal oxide electrodes (expanded titanium and coated with IrO2-Ta2O5) in source-zone groundwater. This work is discussed here rather than as groundwater remediation because the influent 1,4-dioxane concentration was >1,000 mg/L. More than 50% 1,4-dioxane removal was achieved at 10 and 12 volts (V) by six reactors in series (residence time = 1.5 days in each reactor), but the treatment was accompanied by generation of high concentrations of regulated by-products (trihalomethanes and perchlorate). Decreased currents and degradation rates were observed toward the end of the 8.5-month pilot study, indicating the decreased activity of the electrodes.
The main advantage of electrochemical oxidation over AOPs is that it does not require addition of chemicals to generate hydroxyl radicals. However, in practice, production of regulated disinfection by-products and the high costs of electrodes limit its application in full scale.
6.2.4.5 Granular Activated Carbon Adsorption
The removal of 1,4-dioxane by GAC adsorption has been studied more recently. Fotta (Fotta 2012) evaluated 1,4-dioxane removal from contaminated groundwater (average 1,4-dioxane concentration of 2.23 µg/L) by four types of GACs prepared from bituminous coal, lignite, and coconut shells. Results showed that less than 850 BVs of water could be treated with all four GACs before the breakthrough of 30% of the initial 1,4-dioxane concentration occurred.
Kegel et al. (Kegel et al. 2010) reported that 18% of the spiked 1,4-dioxane (initial concentration of 2,000 µg/) was removed by a peat-based GAC filter after 1,200 BVs. The raw water contained 33% groundwater and 67% bank filtrated surface water and was pretreated by RO before passing through the GAC unit. These results were not inconsistent with those from the early work by McGuire et al. (McGuire, Suffet, and Radziul 1978) (Section 6.2.1) because the breakthrough was evaluated at longer EBCTs (3–22 minutes). Together, they indicate that GAC adsorption is unlikely to be feasible for 1,4-dioxane removal in a full-scale application.
Raw material may be an important factor that affects 1,4-dioxane adsorption by GAC. Coconut shell GAC showed the higher capacity for 1,4-dioxane than others in two batch and one column studies [(Curry 2012); (Eigenbrodt and Rooney 2014); (Fotta 2012)]. Similarly, (Johns, Marshall, and Toles 1998) concluded that GAC made from pecan or walnut shells adsorbed more 1,4-dioxane than commercial GACs made of bituminous coal; however, the bed life (i.e., BVs at breakthrough) for even the best-performing GAC type needs to be at least one to two orders of magnitude longer for GAC to be considered cost-effective for full-scale drinking water projects.
To illustrate this point, in the same study where the best-performing GAC registered 850 BVs before 30% breakthrough for 1,4-dioxane (Fotta 2012), TCE and tetrachloroethene (PCE) was not detected in the effluent (i.e., no breakthrough) after 24,000 BVs in all four types of GAC. Thirty percent (30%) breakthrough of 1,1,1-TCA occurred after approximately 8,000 BVs for the GAC type with the lowest adsorption ability. The number of breakthrough BVs shows that while it may be cost-effective to treat 1,1,1-TCA, TCE, and PCE with GAC, it would require much more GAC and frequent GAC changeout to treat 1,4-dioxane in the same water.
6.2.5 Residential Drinking Water Treatment
Treatment options for 1,4-dioxane on the residential level are also limited. The following treatment processes are expected to be ineffective for 1,4-dioxane treatment:
- Commercial pitcher filters and refrigerator filters
- Particulate filters
- UV disinfection
- Water softening
GAC adsorption is known to provide some level of 1,4-dioxane removal if it is designed properly (e.g., adequate contact time) and GAC is changed out frequently. RO membranes used in full-scale water treatment facilities have been shown to remove a portion of 1,4-dioxane (Section 6.2.4.3); therefore, although there appear to be no studies on the removal of 1,4-dioxane by residential RO treatment, it is expected that some treatment may occur.
The following sections further discuss commercial pitcher filters and refrigerator filters (as examples of popular choice of point-of-use treatment [POUT]) and GAC adsorption as a POET treatment approach.
6.2.5.1 Commercial Pitcher Filters and Refrigerator Filters
Commercial pitcher filters and refrigerator filters were found ineffective as POUT for 1,4-dioxane (Knappe et al. 2016). The filtration media in the commercial pitcher filters and refrigerator filters generally rely on activated carbon for organics adsorption. As discussed in Section 6.2.4.5, the adsorption capacity of activated carbon is relatively low. Therefore, as expected, the study found that only 25%–44% of 1,4-dioxane was removed by two commercial filters after 130 L of water were treated. As a comparison, the daily recommended intake volume of water for an adult is approximately 2.7 to 3.7 liters.
6.2.5.2 GAC Adsorption as a POET Treatment Approach
Adsorption by GAC is a common treatment unit available for residential POET systems. Based on 1,4-dioxane’s physical and chemical properties, it does not readily adsorb to GAC (see Section 6.2.4.5 for more detail). However, with sufficient monitoring and changeout frequency, GAC can effectively be implemented for residential water treatment. Some specific considerations for application of GAC include the following:
- GAC type
- Treatment vessel sizing
- Number of treatment vessels
- 1,4-Dioxane monitoring frequency
- GAC changeout frequency
The case study described below highlighting residential GAC treatment is included here.
A GAC system consisting of three 150-pound (Johnson) [68-kilogram (kg)] GAC vessels in series was installed to treat low levels of 1,4-dioxane for a home in North Carolina. Although GAC was anticipated to have low adsorption rates for 1,4-dioxane compared to other VOCs, the treatment performance was achieved by compensating for low adsorption rates with very high GAC to 1,4-dioxane mass ratios (between 70,000 and 160,000 pounds of GAC per pound of 1,4-dioxane removed). The design flow rate was 200 gallons per day and GAC was designed to be exchanged on an annual basis. This equates to an annual requirement of 3,240 lbs of GAC per gallons per minute (gpm) of flow, highlighting why GAC use may become cost prohibitive in larger-scale systems.
Water samples were collected from the influent, midpoint, and effluent sample ports of the POET systems immediately after startup and approximately 1.5 and 3.5 months later. 1,4-Dioxane results were 3.6–3.8 μg/L and nondetect with a method detection limit of 0.3 μg/L in each of the midpoint and effluent water samples collected during the three sampling events. In the following 3 years, the POET system was monitored annually, and the sampling results indicated that 1,4-dioxane was nondetect in the midpoint and effluent.
Treatment vessel sizing and the number of treatment vessels depend on the expected volume of water than can be treated prior to breakthrough. Because 1,4-dioxane will breakthrough GAC more quickly than other compounds, vessels may need to be upsized and/or more vessels may be needed to provide the duration of treatment needed. The monitoring frequency then depends on what the design breakthrough period is. Likewise, GAC changeouts would become dependent on how long each of the vessels performs based on the water usage rate of the residence. For this particular system referenced above, the GAC has been exchanged on an approximate annual basis (as per design). Other maintenance includes changing out the pre-GAC particulate filter monthly.
6.3 Soil/Vadose Zone Treatment
1,4-Dioxane is fully miscible in water, has a low Henry’s law constant, and a low sorption capacity when compared with the chlorinated solvents that may be present as co-contaminants with 1,4-dioxane. Unlike chlorinated solvents, 1,4-dioxane will preferentially dissolve into pore water rather than sorb to the soil matrix or partition into the soil gas. However, there are some instances where vadose zone treatment of 1,4-dioxane may be necessary:
- Landfills
- 1,4-Dioxane manufacturing facilities
- Facilities that store pure phase 1,4-dioxane for use in operations
- Chemical manufacturing facilities where 1,4-dioxane is a generated by-product
- Sites with little infiltration of precipitation (e.g., nonpermeable surface finishes)
- Sites with subsurface physical barrier to migration (e.g., clay lenses, aquiclude/aquitard) s
- Sites with very dry subsurface conditions (e.g., low soil moisture)
1,4-Dioxane’s affinity to be preferentially associated with soil pore water makes it more challenging to remove from unsaturated soils when implementing technologies designed to remove the VOCs frequently released as co-contaminants with 1,4-dioxane. Technologies designed to remove chlorinated solvents from unsaturated soil focused on volatilization (e.g., SVE) or destruction of organic matter within the soil matrix will not be as effective for mass removal of 1,4-dioxane. Conversely, unsaturated soil treatment technologies focused on addressing contaminants present in the pore water will be more effective at removing 1,4-dioxane. The remainder of this section discusses various options for soil/vadose zone treatment, categorized by the technology’s maturity level.
6.3.1 Fully Demonstrated Technologies for Soil
Fully demonstrated technologies are those that have been implemented or demonstrated under full-scale situations and typically include effective, well-documented treatment technologies.
6.3.1.1 Physical Removal (Excavation)
Excavation is a physical removal method that involves digging up and removing contaminated soil matrix from the subsurface. The impacted matrix is either transported off-site for treatment and disposal or treated on-site aboveground and returned to the excavation. Soil sampling is conducted prior to excavation to determine the area and depth of materials requiring removal. Confirmation samples are typically collected from the base and sidewall of the excavation to demonstrate that the impacted media has been removed from the site. Once complete, the excavation is backfilled with clean soil, typically from an off-site source, and compacted at various intervals to minimize adverse impacts to the geotechnical properties of the disturbed area.
Excavation has relatively short active remediation time frames when compared to other treatment methodologies (days to months, depending on the size of the treatment area). The physical removal provided by excavation also provides a high degree of certainty associated with treatment efficacy, so long as the impacted soils can physically be removed (i.e., not hindered by building footers or other limitations). Costs for excavation can be high depending on the concentration of contamination and type of waste, the travel distance to the disposal facility, and the required depth of excavation. Table 6-1 provides a summary of the design characteristics associated with excavation.
Table 6-1. Summary of key parameters for implementation of excavation
| Characteristics | Description |
| Treatment mechanism | Physical removal |
| Treatment location | Typically, off-site |
| Time required for active treatment | Days to months, depending on the size of the treatment area |
| Performance certainty | High—contaminant is physically removed from the site |
| Off-site waste disposal | High volume associated with excavated soils |
| Cost | Depends on waste designation (hazardous vs. nonhazardous), travel distance to disposal facility, and depth of excavation |
| Power requirements | Minimal—limited to powering earth-moving equipment |
| Operation and maintenance | None |
| Treatment depth | Deep excavation is possible; however, this will require larger machinery and/or benching techniques and may require extensive dewatering, which will increase the cost and required time. Excavation is most cost-effective above the water table and to depths up to 9.1 meters (m)/30 feet (ft) below ground surface (bgs). |
| Key design parameters | Area and depth of design treatment volume, excavated waste designation (nonhazardous vs. hazardous), depth to water, and resulting dewatering requirements. |
| Pilot or bench testing recommended | No—not required for effective treatment |
Large earth-moving equipment such as backhoes and excavator trackhoes are used to remove the impacted matrix from the subsurface. The size and type of equipment depends on the area to be excavated and the required depth of excavation. Excavation design and implementation includes designating areas to stage and dewater excavated materials, entrance and egress paths for dump trucks transporting excavated materials off-site, and dust suppression to prevent transport of airborne impacted soil particles. Staging, entrance, and egress methodology is also required to import the clean fill materials that will be used to close the excavation. Air monitoring is conducted to ensure that dust and contaminant vapors are not present at concentrations that may pose a risk to workers.
Costs associated with excavation depend on the area and vertical thickness of the treatment area, the depth to water, and the relative distance to the nearest disposal facility. Excavation below the water table will require additional infrastructure, such as shoring and dewatering, to maintain the excavated area’s integrity. These measures will increase the overall cost of the excavation.
Because 1,4-dioxane will preferentially partition into the pore water within the soil matrix, care should be taken when managing any water generated during excavation activities, including dewatering of the excavation, dewatering of the staged materials after excavation, or managing storm water that has come in contact with impacted materials. Consider the presence and relative concentration of 1,4-dioxane when evaluating dust suppression methods. Because of 1,4-dioxane’s affinity to dissolve in water, dust suppression methodologies that involve application of water to the treatment area may result in the generation of a new 1,4-dioxane-impacted waste stream that requires proper disposal.
6.3.1.2 Ex Situ Thermal Desorption
Excavated soils can be treated ex situ on site with commercially available mobile thermal desorption units. Thermal desorption is a thermally induced separation process. It is not intended to destroy contaminants, but rather physically separates the volatilized contaminants and water vapor from the soil matrix. Thermal desorption can be implemented as low-temperature thermal desorption (LTTD) or high-temperature thermal desorption (HTTD).
LTTD is applied to contaminants with boiling points less than 600 °F. In an LTTD process, the waste stream is heated to temperatures between 300°F and 600°F. LTTP is typically applied for soils impacted with VOCs. HTTD was developed for contaminants with a boiling point higher than 600°F. HTTD involves heating the waste stream to temperatures between 600°F and 1,200°F and is required when more recalcitrant contaminants, such as SVOCs, pesticides, PCBs, creosote, or coal tar, are present. Excavated soil treated with thermal desorption will retain basic physical properties and can be used as backfill. Table 6-2 provides a summary of the design characteristics associated with ex situ thermal desorption.
Table 6-2. Summary of key parameters for implementation of thermal desorption
| Characteristics | Description |
| Time required for active treatment | Days to months, depending on the size of the treatment area and the contaminant concentration |
| Treatment location | Ex situ |
| Performance certainty | Moderate to high—excavation and blending of the soil prior to treatment create a more uniform treatment matrix, which results in increased removal efficiency |
| Off-site waste disposal | Minimal volume because extracted contaminants are condensed to a small volume of liquid waste or destroyed via thermal oxidation as part of the treatment process |
| Cost | Moderate to high depending on power requirements, excavation depth, and dewatering requirements |
| Power requirements | Fuel (frequently natural gas) and electrical source required to operate thermal desorption unit and off-gas treatment |
| Operation and maintenance | Active O&M required during system operation |
| Treatment depth | Limited by achievable depth of excavation; if excavation is completed below the water table, some dewatering may be required prior to the thermal desorption process to decrease the soil moisture content |
| Key design parameters | Area and depth of design treatment volume, mass removal requirements, contaminant suite |
| Pilot or bench testing recommended | Yes—bench testing recommended to determine optimal residence time and design temperature |
During the thermal desorption process, soil is heated to volatilize water and organic contaminants. The volatilized contaminants and water vapor are removed from the system by applying a vacuum or carrier gas. The heating temperatures and residence times are designed to volatilize the design contaminant but are not designed to oxidize or destroy them.
Thermal desorption is applied as a two-step process. During the first step, the excavated impacted soil material is heated to volatilize contaminants and vaporize soil moisture, which separates the contaminants from the soil particles. The excavated impacted soil is placed in the desorption unit and heated to the design temperature for a specific amount of time. The required temperature and residence time will depend on the contaminant suite requiring treatment, the soil type, and the moisture level of the impacted matrix.
During the second step, the volatilized contaminant and water vapor stream separated from the solid matrix as a result of the heating process is removed from the thermal desorption unit either by applying a vacuum or through flow of a carrier gas. The separated vapor waste stream is routed to a vapor treatment unit to remove, capture, or destroy the contaminants. The vapor waste stream can be treated by several processes, including thermal oxidation, condensation, filtration, or sorption.
Thermal desorption is an ex situ treatment, so impacted materials must be excavated, separated, and staged for aboveground treatment. Large rocks and debris must be separated from the waste stream or crushed prior to treatment to maximize uniform heating during treatment. Very wet soils will not heat properly, and dewatering may be required to reduce moisture content prior to treatment, depending on the saturation level of the excavated soil.
Based on the boiling point of 1,4-dioxane, LTTD will provide sufficient heating to separate 1,4-dioxane from the soil matrix. 1,4-Dioxane has an affinity for water, and as a result may partition into the condensate preferentially over the gas stream during vapor treatment. 1,4-Dioxane in water will not sorb to GAC with the same efficiency as other VOCs because of its low sorption capacity; as such, GAC may not provide an effective means of treating the liquid waste streams that result from thermal desorption. Similar to excavation, care should be taken during any dewatering that is required prior to the thermal desorption process to contain and treat 1,4-dioxane impacted groundwater and/or storm water.
6.3.1.3 Solidification and Stabilization
Solidification is a physical immobilization process whereby contaminants are entrapped within the soil matrix by encapsulating contaminated soil particles within a low-permeability solid material. Stabilization is a chemical immobilization process in which chemical reactions between the impacted material and the stabilization reagent alter the properties of the impacted material so that the contaminants can no longer leach out of the matrix. Solidification and stabilization will decrease the potential for contaminant migration from the unsaturated zone to groundwater by reducing the surface area exposed to percolating water. Solidification and stabilization are not destructive processes. The contaminant mass remains in place; it is just immobilized within the soil matrix. Table 6-3 provides a summary of the design characteristics associated with solidification and stabilization.
Table 6-3. Summary of key parameters for implementation of solidification and stabilization
| Characteristics | Description |
| Time required for active treatment | Days to months, depending on the size of the treatment area and the contaminant concentration |
| Treatment location | Typically, in situ |
| Performance certainty | Moderate to high—excavation and blending of the soil prior to treatment create a more uniform treatment matrix, which results in increased removal efficiency |
| Off-site waste disposal | Minor volume, since a limited volume of surficial soil must be removed to account for bulking during the mixing operation |
| Cost | Cost depends on amendment required and depth of treatment |
| Power requirements | Minimal—fuel for earth-moving equipment |
| Operation and maintenance | Occasional monitoring may be required following active treatment to confirm the contaminants remain immobilized |
| Treatment depth | Limited by achievable depth of mixing equipment |
| Key design parameters | Area and depth of design treatment volume, required additive recipe |
| Pilot or bench testing recommended | Yes—bench testing recommended to determine optimal mix design for immobilization based on soil type and contaminant suite |
The solidification and/or stabilizing agent is blended or mixed into the subsurface by augers, excavators, or specialized rotary equipment that can break up the soil column and allow the binding agent to come in contact with contaminants sorbed to soil. Common solidification and/or stabilization agents that are also applicable to 1,4-dioxane remediation include Portland cement, asphalt, fly ash, lime, and clay. Note that addition of these agents results in an increase in total material volume.
Solidification/stabilization is frequently conducted in situ, and the resulting low-permeability mixture not only immobilizes contaminants present in the unsaturated zone but also acts as a cap to prevent infiltration of rainwater to the potentially contaminated matrix below the treated area. The solidification/stabilization process leaves the contaminated matrix in place and as a result may require long-term monitoring and maintenance to document the continued effectiveness of the remedy.
Because 1,4-dioxane will preferentially partition into water (i.e., low sorption to soil), an important design consideration will be the development of a mix design that eliminates or minimizes soil moisture and overall permeability of the treated area. Management of water during mixing operations will also need to be carefully considered. Disposal of wastewater generated during dewatering activities that take place during mixing operations will need to be carefully managed.
6.3.1.4 Phytoremediation
Phytoremediation is a demonstrated technology that can be used to treat 1,4-dioxane in unsaturated soil as well as the saturated soil matrix. A description of phytoremediation implementation for treatment of 1,4-dioxane can be found in Section 6.5.1.3.
6.3.2 Emerging Options for Soil
Emerging options are technologies that may be partially demonstrated or researched and may include technologies implemented under laboratory bench-scale or pilot-scale situations. Typically, less documentation, research, or validation is available.
6.3.2.1 Extreme Soil Vapor Extraction
Traditional SVE involves the physical stripping of volatile contaminants from the unsaturated soil matrix by applying a vacuum to increase the air flow rate within the subsurface and promote volatilization. Air pulled through the soil matrix promotes desorption of contaminants bound to the soil particles and dissolved in soil moisture into the vapor phase. Although some removal of 1,4-dioxane by conventional SVE systems occurs, 1,4-dioxane is difficult to remove by traditional SVE systems because it is sequestered in the pore water.
Extreme (or enhanced) SVE (XSVE) is an enhancement of traditional SVE to increase 1,4-dioxane removal rates through decreased infiltration, increased air flow, focused vapor extraction, and/or injection of heated air. Because XSVE is an emerging treatment technology, the definition of what qualifies as XSVE has not been firmly established beyond the enhancement of traditional SVE through added heat, increased air flow, injected air flow, more closely spaced injection and extraction points, or other modifications to traditional SVE that allow more aggressive extraction of contaminants from the unsaturated soil matrix. Because the removal mechanisms associated with XSVE are more aggressive than traditional SVE, XSVE will also result in treatment of other VOCs present as co-contaminants with 1,4-dioxane. Table 6-4 provides a summary of the design characteristics associated with XSVE.
Table 6-4. Summary of key parameters for implementation of XSVE
| Characteristics | Description |
| Treatment mechanism | Physical separation through enhanced volatilization or vaporization of soil moisture |
| Treatment location | In situ |
| Time required for active treatment | Months to years |
| Performance certainty | Moderate—technology is still in pilot-test phase |
| Off-site waste disposal | Minimal volume, since extracted contaminants are condensed into a concentrated waste stream |
| Cost | Moderate, depending on O&M and power requirements |
| Power requirements | Active power source required for heating and operation of mechanical system during operation |
| Operation and maintenance | Active O&M required during operation of the XSVE system |
| Treatment depth | Limited by depth to groundwater |
| Key design parameters | Permeability of site-specific geology, soil moisture content, ease of installation, and operation of a mechanical system |
| Pilot or bench testing recommended | Pilot testing recommended; technology is still under development |
XSVE is designed to modify the subsurface characteristics to increase the mobility and volatility of 1,4-dioxane and improve extraction from the subsurface. Removal rates for 1,4-dioxane during operation of a traditional SVE system are limited by 1,4-dioxane’s low Henry’s law constant at ambient temperatures and the preferential partitioning of 1,4-dioxane into the pore water rather than the soil vapor. The Henry’s law constant for 1,4-dioxane is temperature dependent and increases with increasing temperature. XSVE applies an increase in subsurface temperature to increase the volatility of 1,4-dioxane.
Traditional SVE systems increase air flow through the subsurface by applying a vacuum at the extraction points and allowing ambient air to be pulled into soil matrix as a result of the inherent permeability in the ground surface. XSVE involves pushing heated air throughout the treatment area at several injection points in addition to pulling a vacuum on the subsurface at the extraction points. Injection of air during XSVE not only increases the air volume exchange rate in the treatment area, which will increase the potential volatilization of 1,4-dioxane, but may also remove 1,4-dioxane from the subsurface by extracting 1,4-dioxane dissolved in pore water.
Because XSVE is an emerging technology, limited field data has been collected to refine the pertinent design parameters, and the data that has been collected is largely related to bench and pilot testing. Hinchee et al. (Hinchee et al. 2018) conducted an XSVE pilot test at McClellan Air Force Base in California as part of an Environmental Security Technology Certification Program (ESTCP) project (Project Number ER201326). Table 6-5 summarizes data collected during the pilot test and the design parameters for a traditional SVE system implemented at the same site.
Table 6-5. XSVE pilot-test data
Adapted from Hinchee et al. 2018
| Parameter | Traditional SVE | XSVE |
| Injection wells | No | Yes |
| Extraction wells | Yes | Yes |
| Injection well spacing | — | 6.1 m (20 ft) grid |
| Extraction well spacing | 35 to 40 ft | <4.6 m (<15 ft) from injection well |
| Injection flow rate per well | — | 1.9 to 2.5 standard cubic meters per minute (m3/min) (70 to 90 standard cubic feet per minute [scfm]) |
| Extraction flow rate per well | 1.1 to 2.2 m3/min (40 to 80 scfm) | 2.0 to 3.1 m3/min (70 to 110 scfm) |
| Injection temperature | — | 100°C to 130°C |
| Extraction temperature | Ambient | Less than 40°C |
| Treatment area temperature | Ambient | 40°C to 90°C |
| Extraction wells | Yes | Yes |
A pore volume exchange rate of approximately 20,000 pore volumes was achieved over the 14-month duration of the pilot test. Performance monitoring data demonstrated a 94% reduction in 1,4-dioxane concentration and a 45% reduction in soil moisture. XSVE flow rates will be highly dependent on geology. The pilot test described above was conducted in primarily sandy/silty sand soil at a site in California.
More research is needed to determine which adjusted design parameter has the biggest impact on 1,4-dioxane removal efficiency. It is unclear if increased air flow, increased temperature, or reduction in soil moisture is the primary mechanism for the improved removal efficiency of XSVE when compared to traditional SVE. Similar to traditional SVE, design parameters for XSVE will vary based on site-specific conditions, including geology, soil permeability, and moisture content.
Location of contaminant mass in the subsurface is a key design parameter, and distribution of 1,4-dioxane in the subsurface can be more limited than the distribution of chlorinated solvents. A detailed sampling program should be complete prior to implementation of XSVE to ensure the zones with the highest mass of 1,4-dioxane are being targeted for treatment.
Injection of heated air helps volatilize water and consequently remove 1,4-dioxane dissolved in water; however, it is possible for the water vapor to recondensate before it is removed from the subsurface, resulting in a redistribution of 1,4-dioxane in the soil matrix as opposed to the desired mass removal through extraction. If a sufficient volume of water is recondensed in the same area, this could result in a vertical migration of 1,4-dioxane impacted water. There is also potential for 1,4-dioxane to contaminate clean soil if 1,4-dioxane redistributes into the pore water of a previously clean area.
Materials compatibility should be evaluated during the design phase to ensure the elevated temperatures associated with XSVE will not adversely impact operation. For example, the melting point of polyvinyl chloride (PVC) ranges from 100°C to 260°C, depending on the manufacturer additives. Alternative materials such as stainless-steel piping should be considered during system design in areas where elevated temperatures are expected.
Treatment of the extracted vapor and condensate stream from an XSVE system will be a key design consideration. Because 1,4-dioxane will preferentially dissolve into water rather than volatilize, 1,4-dioxane will concentrate in the condensate from the XSVE system over being released as part of the vapor emissions stream. Any 1,4-dioxane that is emitted to the atmosphere as part of the vapor emissions from an XSVE system will be readily oxidized by sunlight. 1,4-Dioxane in water will not sorb to GAC with the same efficiency as other VOCs because of its low sorption capacity; as such, GAC may not provide an effective means of treating liquid condensate waste streams.
6.3.2.2 In Situ Chemical Oxidation Soil Blending
In situ chemical oxidation (Barajas-Rodriguez and Freedman 2018) soil blending is a method of distributing oxidant amendment through the soil matrix by physically disturbing the treatment area with a mechanical mixer. Soil blending helps to break up the soil column, exposing contaminant mass that may be sorbed to soil and/or trapped in microfractures containing pore water. This allows for a more uniform distribution of amendments throughout the treatment area.
The improved contact between the oxidant and contaminant mass results in an overall improvement in oxidant treatment efficacy. Soil blending can be effective in both the saturated and unsaturated zone for various soil types, including sands, gravels, silts, and clays, although each soil type comes with specific design considerations. Table 6-6 summarizes the design characteristics associated with ISCO soil blending.
Table 6-6. Summary of key parameters for implementation of ISCO soil blending
| Characteristics | Description |
| Treatment mechanism | Destruction through chemical oxidation |
| Treatment location | In situ |
| Time required for active treatment | Weeks to months |
| Performance certainty | Moderate—technology is still in pilot-test phase |
| Off-site waste disposal | Minimal cost/volume, since some bulking may occur during mixing that will require off-site disposal of excess soil |
| Cost | Moderate at depths between 6.1 to 9.1 m bgs (20 and 30 ft bgs); high at treatment depths greater than 9.1 m bgs (30 ft bgs) |
| Power requirements | Minimal—fuel for mixing equipment |
| Operation and maintenance | None once treatment is complete |
| Treatment depth | 0 to 30 m bgs (0 to 100 ft bgs) |
| Key design parameters | Treatment depth, depth to water, contaminant type, background oxidant demand |
| Pilot or bench testing recommended | Bench testing to determine optimal design mix |
Soil blending is typically conducted with either a large-diameter auger rig or a rotary drum blender mounted on the end of an excavator arm. Large-diameter auger rigs mix a fixed area of soil by spinning an auger to the desired depth. Once an area has been sufficiently mixed, the rig is moved to treat the next column of soil. Large-diameter auger rigs come in multiple sizes, with the largest treating an area up to 3.0 m (10 ft) in diameter at a time. The primary advantage of a large-diameter auger rig is the ability to mix unsaturated and saturated soil to depths greater than 30 m bgs (100 ft bgs). Large overhead clearance can be required for large-diameter auger rigs, depending on the treatment depth.
Rotary drum blenders are more agile in mixing shallow soil over a larger area than large-diameter auger rigs; however, rotary drum blenders are limited by depth. The mixing depth for a rotary drum blender is limited by the length of the excavator arm. Rotary drum blenders can be used at depths up to approximately 6 m bgs (20 ft bgs) without benching. Benching and other engineering controls can allow rotary drum blenders to be effectively implemented at greater depths. The depth to the water table will impact the overall depth to which a rotary drum blender can be safely implemented.
The addition of the liquid volume associated with the oxidant amendment in combination with the soil mixing process will decrease the soil column’s structural stability. A stabilizing agent such as Portland cement can be incorporated into the design to increase the treatment area’s load-bearing capacity post-treatment.
Selection of an oxidant is an important component of ISCO soil blending. The primary oxidants used for ISCO soil blending, permanganate and activated persulfate, can be effective for treatment of 1,4-dioxane under the right conditions. The oxidant chemistry associated with ISCO soil blending is similar to the oxidant chemistry for delivery of an oxidant via in situ injection. Information regarding in situ oxidation of 1,4-dioxane is provided in Section 6.5.1.1.
Similar to in situ injection of an oxidant, the contaminant concentration, oxidant kinetics, treatment area and associated volume of groundwater, and the background oxidant demand are key design parameters to determine the required oxidant dosing. For liquid oxidants, enough contact time must occur for the reaction to take place; soil mixing and addition of water can be helpful in maximizing contact time. Bench testing is recommended prior to implementation to determine the optimal oxidant recipe for full-scale implementation. Due to the cost of mobilizing the mixing equipment, pilot tests typically are not conducted for ISCO soil blending.
6.3.2.3 In Situ Thermal Remediation
In situ thermal remediation (ISTR) is an established technology for in situ treatment of chlorinated solvents. Recent research related to use of ISTR for treatment of 1,4-dioxane indicates in situ thermal treatment can be an effective means of treating 1,4-dioxane in unsaturated soil as well as in the saturated soil matrix. A description of thermal remediation implementation for treatment of 1,4-dioxane can be found in Section 6.5.2.
6.3.3 Less Effective Technologies for Soil
Less effective technologies are just that, and typically include technologies with negligible or limited capability of 1,4-dioxane removal based on demonstration sites and/or theoretical considerations from 1,4-dioxane properties.
Several unsaturated soil remediation technologies that can be effectively implemented for co-contaminants typically found with 1,4-dioxane including chlorinated ethenes and ethanes are not effective for treatment of 1,4-dioxane. A brief description of select unsaturated soil treatment technologies that are not effective for 1,4-dioxane is provided below.
6.3.3.1 Traditional SVE
As discussed in Section 6.3.2.1, traditional SVE relies on preferential partitioning of contaminants into the vapor phase to facilitate the primary removal mechanism. Because 1,4-dioxane has a lower volatility than other VOCs, which can be effectively treated with SVE, and because 1,4-dioxane will preferentially partition into the pore water within the soil matrix, enhancements to traditional SVE systems are required for effective removal of 1,4-dioxane, except under specific site conditions (e.g., arid climate). Implementation of an enhanced SVE system for removal of 1,4-dioxane, or XSVE, is discussed in Section 6.3.2.1.
6.3.3.2 Biodegradation
Traditional soil treatment technologies that reduce contaminant mass through enhanced biodegradation include in situ bioventing and ex situ bio-piles. Both technologies rely on the introduction of air flow into the contaminated matrix to increase the oxygen available to promote aerobic biodegradation. Metabolic aerobic biodegradation of 1,4-dioxane in the vadose zone is not well documented. Vadose zone biodegradation may be possible under less-traditional engineered conditions, such as those described in Section 6.5.1 and 6.5.2.
6.4 Ex Situ Groundwater Treatment
Ex situ groundwater treatment processes require groundwater extraction as an initial step. Options for treatment of extracted groundwater are discussed in following sections.
6.4.1 Fully Demonstrated Technologies for Groundwater (Ex Situ)
Fully demonstrated technologies are those that have been implemented or demonstrated under full-scale situations and typically include effective treatment technologies that are well documented.
6.4.1.1 Advanced Oxidation Processes
AOPs have been discussed in the context of drinking water treatment in Section 6.2.4.1. The major design considerations for ex situ groundwater treatment are similar to those for drinking water, although the 1,4-dioxane concentration, flow rate, and intrinsic water quality may differ. 1,4-Dioxane concentrations in drinking water sources rarely exceeds 30 μg/L (Adamson, Piña, et al. 2017), but the range of 1,4-dioxane in contaminated groundwater can be much wider.
While most municipal drinking water wells are pumped at hundreds of gpm or higher, many remediation systems have smaller flows. In addition, the water quality may be more challenging for groundwater remediation projects, which may impact the selection of specific AOP technologies or require additional treatment process prior to and/or after the AOP system. For example, GAC may be needed for additional VOC removal after AOP, or a process to remove reduced metals may be needed before AOP. A case study of AOP application to groundwater treatment can be found here.
6.4.1.2 Sorptive Resins
Sorptive resins are also demonstrated technologies for 1,4-dioxane removal from water. As an example, AMBERSORBTM 560 (AMBERSORB) is a carbonaceous adsorbent manufactured by the partial pyrolysis of a sulfonated cationic styrene-divinylbenzene copolymer. AMBERSORB has a high surface area and engineered porosity and is a hard, nondusting spherical adsorbent. Due to the engineered porosity and particle size distribution, AMBERSORB has 5 to 10 times the sorption capacity as GAC and can operate at significantly shorter EBCT than GAC (Woodard, Mohr, and Nickelsen 2014). It can be regenerated in place using low-pressure steam.
Similar to GAC, sorptive resins are typically implemented in multiple vessels in series (lead-lag operation, as depicted here). After a predetermined loading cycle duration, or after breakthrough to a specified level is observed from the lead vessel, the lead vessel is taken offline for regeneration. The lag vessel is placed in the lead position and a third standby vessel is placed in service in the lag position. The offline bed is regenerated by low-pressure superheated steam before it is placed in the standby position. The highly concentrated condensate from the steam regeneration is often treated through a small GAC vessel to minimize the volume of waste to be disposed. This approach takes advantage of the greater 1,4-dioxane adsorption capacity of GAC at elevated concentrations. These operations can be fully automated.
AMBERSORB has successfully demonstrated the effective removal of 1,4-dioxane over a wide range of concentrations and operating conditions, including those created by ISTR. 1,4-Dioxane can be consistently treated to nondetect levels (<0.3 µg/L). A case study for AMBERSORB is included here.
In general, sorptive resin systems may have a higher capital cost than AOPs, partly due to the cost of the resin. However, the advantage is the streamlined operation and potentially lower O&M compared to AOPs. Other limitations of sorptive resins include its nondestructive nature and the generation of a waste stream. The impact of more complex water matrices (e.g., high NOM) on their performance is less well understood (Bell and Forsberg 2019).
In theory, sorptive resin can also be used for POET, but at the time of this writing, it has not been certified for this use. A potential drawback may be its high cost compared to other POET systems.
6.4.2 Emerging Options for Groundwater (Ex Situ)
Emerging options are technologies that may be partially demonstrated or researched and may include technologies implemented under laboratory bench-scale or pilot-scale situations. Typically, less documentation, research, or validation is available.
6.4.2.1 Biological Treatment
Bioreactors for 1,4-dioxane removal in industrial wastewater and landfill leachate have been described in Section 6.2.3. To reiterate a few key points, the 1,4-dioxane concentration, water quality matrix, and treatment standards are vastly different in typical contaminated groundwater than in industrial wastewater.
The best treatment performance previously obtained from a bioreactor was in a cometabolic system with THF as the primary substrate. A concentration of 200 μg/L of 1,4-dioxane was treated to 9 μg/L in a trickling filter at a THF/1,4-dioxane mass ratio of 110:1 (Zenker, Borden, and Barlaz 2004). The only full-scale biological system for 1,4-dioxane treatment reported thus far is also thought to be based on a cometabolic process (THF/1,4-dioxane ratio = 2 to 3), which achieved an effluent concentration of just below 100 μg/L (Cordone et al. 2016). In this case, the THF was fortuitously present in the landfill leachate. However, with the anticipated difficulty in obtaining acceptance for THF addition, or addition of other primary substrates with groundwater standards, the only remaining options for implementing biological treatment are metabolic degradation or using a nontoxic substrate such as an alkane gas.
To date, no biological treatment system has been able to consistently meet the relatively low regulatory standard concentrations (e.g., 3 μg/L or lower); therefore, a significant breakthrough needs to be made for it to be considered an established remediation technology for ex situ groundwater treatment.
6.4.2.1.1 Bioreactors Using Metabolic Degradation
A review of the half-saturation concentration (Priddle and Jackson 1991) of the reported 1,4-dioxane-metabolizing strains isolated so far has led to the notion that metabolic biological treatment lacks the inherent ability to support the growth of metabolic 1,4-dioxane-degrading bacteria at concentrations at or close to the regulatory standards [(Barajas-Rodriguez and Freedman 2018);(McElroy and Hyman 2019)]. However, new and more efficient metabolic degrading microbes may be discovered in the future, or better performance of metabolic treatment systems may be achieved by using a mixed culture selected for conditions mimicking continuous bioreactor operation. Sock (Sock 1993) showed that the degradation kinetic characteristics (particularly the Ks) of a mixed culture can be significantly improved after being “selected” in a continuously diluted bioreactor with 1,4-dioxane as the sole carbon and energy source. The Ks after the selection was on the order of 1 mg/L, albeit at a relatively high temperature of 35°C.
The performance of a metabolic bioreactor can also be enhanced through bioreactor engineering. Sandy et al. (2001) provided an example of using multiple stages of aerobic bioreactors, and the effluent in the pilot system reached 40 μg/L from 430 mg/L in the influent (see more details in Section 6.2.3.1). Another possible improvement is through the use of a biofilm reactor, such as biological granular activated carbon (BioGAC). BioGAC was successfully applied to treat methyl-tertiary-butyl ether (MTBE) in the past, an emerging contaminant at the time with similar physical and chemical properties to 1,4-dioxane (Sun et al. 2003). Using a pure culture in recirculated batch studies at a high 1,4-dioxane concentration, Myers et al. (Myers et al. 2018) showed that BioGAC removed 99% (from 73.0 to 0.800 mg/L) and 94% (from 3.6 to 0.220 mg/L) of 1,4-dioxane in wastewater and groundwater, respectively.
6.4.2.1.2 Bioreactors Using Cometabolic Degradation with Alkane Gases
Based on the kinetic parameters (Barajas-Rodriguez and Freedman 2018) and successful pilot tests in in situ bioremediation [(Chu et al. 2018); (Lippincott et al. 2015)], cometabolic bioreactors using propane are expected to be effective in treating 1,4-dioxane. Other potential alkane primary substrates include ethane, butane, and isobutane [(Hatzinger et al. 2017); (Krippaehne 2018); (Rittmann et al. 2019); (Xiong et al. 2020)].
There have been several encouraging studies on cometabolic bioreactors using alkanes for 1,4-dioxane treatment. Horst et al. (Horst et al. 2019) pilot tested a propane-fed bioreactor over a period of 2 months. The bioreactor was fed with approximately 0.07 kg per day (0.15 lb per day) of propane (cycled 30 minutes on/30 minutes off); approximately 0.5 to 1 L per minute of pure oxygen (standard grade); and macro- and micronutrients. The bioreactor was seeded with propanotrophic Rhodococcus ruber strain ENV425. During operation, oxygen concentrations in the bioreactor were up to 31.6 mg/L, and propane concentrations were up to 19 mg/L. At peak operation of approximately 2.5 weeks, 1,4-dioxane removal was approximately 83%—from 240 µg/L influent to 40 µg/L effluent.
Rittmann (Rittmann et al. 2019) described a bench bioreactor system that achieved significant 1,4-dioxane removal with a mixed culture using presaturated ethane (58 mg/L, achieved via sparging of pure ethane gas). Oxygen was supplied via diffusion through a membrane. The influent 1,4-dioxane concentration of 44 mg/L was removed by 99% at steady state for over 4 weeks. However, the high 1,4-dioxane concentration and long HRT (13 hours) were not relevant for groundwater remediation, so the author provided a mathematical model to show 1,4-dioxane could be treated to below 1 μg/L with this technology.
Xiong et al. (Xiong et al. 2020) studied a similar bioreactor configuration in which alkane primary substrates (as opposed to oxygen) were delivered through a membrane. Four alkane gases (methane, ethane, propane, and butane) and ethene were used as the primary substrates, with helium as the control. The influent 1,4-dioxane concentration was approximately 600 μg/L. Of the gaseous substrates tested, only ethane and propane promoted 1,4-dioxane removal relative to the control reactor. At steady state, approximately 75% of the 1,4-dioxane was removed when ethane was supplied, compared to 50% under propane. In the case of ethane, the treatment performance was sustained for >300 days. The effluent ethane concentration at steady state was 0.01 mg/L, suggesting both rapid ethane uptake and efficient gas delivery by the membrane.
The type of alkane, the safe delivery of a combustible gas, the control of alkane/1,4-dioxane ratio to prevent inhibition by the primary substrate, and maintenance of sufficient oxygen are likely key design and engineering considerations in the advancement and scale-up of this ex situ treatment technology.
6.4.2.2 Electrochemical Treatment
Section 6.2.4.4 discussed electrochemical treatment as an emerging technology for 1,4-dioxane treatment in industrial wastewater. The same treatment principles can be applied to ex situ groundwater treatment. To reiterate, Blotevogel et al. (Blotevogel et al. 2019) presented the first pilot study using electrochemical treatment to treat source-zone 1,4-dioxane at concentrations above 1,000 mg/L. The main advantage of electrochemical oxidation over AOPs is that it does not require addition of chemicals to generate hydroxyl radicals. However, in practice, production of regulated disinfection by-products and the high costs of electrodes limit its application in full scale.
6.4.3 Less Effective Technologies for Groundwater (Ex Situ)
Less effective technologies are just that, and typically include technologies with negligible or limited capability of 1,4-dioxane removal based on demonstration sites and/or theoretical considerations from 1,4-dioxane properties.
The following treatment technologies are ineffective in 1,4-dioxane treatment for ex situ groundwater remediation (see Sections 6.2.1 and 6.2.5 for additional information):
- GAC adsorption
- Air stripping
- Ion exchange
- Ozonation alone
6.5 In Situ Groundwater Treatment
In situ groundwater treatment does not require groundwater extraction as a preliminary step. Options for in situ groundwater treatment are discussed in the following sections.
6.5.1 Fully Demonstrated Technologies for Groundwater (In Situ)
Fully demonstrated technologies are those that have been implemented or demonstrated under full-scale situations, and typically include effective treatment technologies that are well documented.
6.5.1.1 In Situ Chemical Oxidation
ISCO is a remediation technology in which oxidants are delivered to the subsurface to chemically convert hazardous compounds to nonhazardous or less toxic compounds that are more stable, less mobile, or inert. Redox reactions involve the transfer of electrons from one compound to another. Specifically, one reactant is oxidized (loses electrons) and one is reduced (gains electrons). The oxidizing agents most commonly used for treatment of hazardous contaminants in soil and groundwater are hydrogen peroxide, catalyzed hydrogen peroxide, potassium permanganate, sodium permanganate, sodium persulfate, potassium persulfate, and ozone. Each oxidant has advantages and limitations, and while applicable to soil contamination and some source-zone contamination, they have been applied primarily toward remediating groundwater (www.clu-in.org).
This document presents some background on ISCO, but the primary focus is on ISCO for treatment of 1,4-dioxane. Additional information on ISCO can be found in ITRC’s Technical and Regulatory Guidance for In Situ Chemical Oxidation of Contaminated Soil and Groundwater, Second Edition (ISCO-2 (ITRC 2005)) and other resources (e.g., (Siegrist 2011)). Note that some of the information in these resources may now be outdated.
6.5.1.1.1 Sodium and Potassium Persulfate
Persulfate salts dissociate in water to yield the persulfate anions (S2O8 2 ⎯). Common persulfate salts include sodium persulfate, potassium persulfate, and ammonium persulfate. The sodium form of persulfate has a high solubility (50+%) and a relatively long track record for use in ISCO. The potassium form has much lower solubility (approximately 3%). Potassium persulfate has a much lower solubility in water; its solubility at 25°C is only 6%, versus 40% solubility for sodium persulfate (Table 1-3; (ITRC 2005)). Therefore, potassium persulfate is used for slow-release applications, whereas sodium persulfate is used for fast-acting ISCO applications. Ammonium persulfate is not commonly used because it may lead to the formation of ammonia, which is regulated in groundwater. The chemistry discussion in this section is theoretically applicable to both forms.
The persulfate anion is a strong oxidant (more powerful than the hydrogen peroxide oxidant, for example). However, it is kinetically slow in destroying 1,4-dioxane and other organic chemicals. The oxidation kinetics can be increased by using heat, ferrous or chelated iron, hydrogen peroxide, or increased alkalinity (high pH), which are referred to as “activators.” The main objective of the activation process is to promote the formation of free radical species that are more reactive with organic compounds than the persulfate anion. The free radicals that are formed depend on the activation mechanism that is used. They can include the sulfate radical (SO4⎯ •, an oxidizing radical), the hydroxyl radical (OH⎯ •, an oxidizing radical), and, in the case of alkaline-activated persulfate, the superoxide radical (O2 •─, a reducing radical; (Furman 2010)).
Due to the presence of an unpaired electron, these free radical species are very reactive but also short-lived and, when formed, expedite the destruction of 1,4-dioxane and other organic chemicals. Both the sulfate radical and the hydroxyl radical are potent oxidizing agents that can destroy 1,4-dioxane. Sulfate-free radicals have a reported half-life of about 4 seconds under elevated temperature conditions (≅40°C) (Banerjee and Konar 1984). Hydroxyl radicals have a somewhat shorter half-life because they are kinetically faster.
The term “catalyst” has been used in the past when describing activators, but this term is typically incorrect from a technical perspective, as the activator typically is consumed by the chemical reaction and thus is not technically a catalyst. The activator must be distributed and transported with the persulfate. As with all oxidants, the optimal oxidant loading, including both target and nontarget compounds (due to site-specific matrix demand), should be determined before injection. As with all oxidants, metals can be mobilized within the treatment zone due to a change in oxidation states and/or pH.
Persulfate chemistry is also interrelated with the groundwater system’s pH. Persulfate decomposition produces acid, so persulfate treatment will reduce the system’s pH, all else being equal. The magnitude of this effect varies depending on site-specific conditions such as buffering capacity. At a lower pH, iron may become more soluble, enhancing persulfate activation. At a lower pH, persulfate can hydrolyze to form peroxymonosulfate (HSO5–, or Caro’s acid) (Petri et al. 2011). At a higher pH, the relative proportion of hydroxyl radicals formed is greater, resulting in the ability of such a system to treat a wider range of organic compounds.
Activation with ferrous iron
The activation of persulfate with ferrous iron (Fe[II]) is effective at generating sulfate radicals in solution that are capable of degrading 1,4-dioxane. However, excess ferrous iron in solution will scavenge sulfate radicals. As with Fenton’s reactions, under neutral-to-high pH and/or more highly oxidizing conditions, Fe(II) will be oxidized to ferric iron (Fe[III]) and precipitate out of solution, making it unavailable for persulfate activation. At a pH less than 5, Fe(II) is reconverted to Fe(III), which traditionally was the objective for ISCO applications. Chelation agents can be used to aid in maintaining Fe(II) solubility and transport in the subsurface by complexing with iron in solution [(Liang et al. 2003a); (Liang et al. 2003b); (Liang et al. 2003c)]. Additional details regarding the application and chelation of ferrous iron for the activation of persulfate can be found in previous ITRC documentation (ITRC 2005).
Fe(II) activation tends to generate oxidative radicals, particularly the sulfate radical (Petri et al. 2011). Fe(II) activation directly leads to the generation of hydroxyl radicals, although they may be formed from the interaction between the sulfate radical and water. This activation mechanism is not generally reactive with chlorinated ethanes and methanes that may be commingled with 1,4-dioxane. If 1,4-dioxane is comingled with such contaminants, alternative activation methods such as alkaline activation may be considered based on the likelihood of generating additional oxidative radicals, such as the superoxide radical (e.g., see alkaline activation).
Alkaline activation
Another approach to activate persulfate is the use of alkaline conditions [i.e., pH greater than 10.5 (ITRC 2005)]. Alkaline-activated persulfate forms oxidative radical species, reductants, and nucleophiles (Furman, Teel, and Watts 2010). This multiradical attack allows the treatment of chlorinated ethenes (TCE, PCE, DCE, and vinyl chloride [VC]); 1,4-dioxane; MTBE; tert-butyl alcohol (TBA); petroleum hydrocarbons (benzene, toluene, ethylbenzene, and xylene [BTEX] and PAHs); pesticides; and more recalcitrant compounds, including chlorinated methanes (such as carbon tetrachloride) and chlorinated ethanes (such as 1,1,1-TCA).
Basic conditions increase the production of hydroxyl free radicals due to the reaction of the hydroxide ion and other radicals. Hydrated lime, calcium hydroxide, and sodium hydroxide are three typical alkaline reagents that have been used in laboratory and field applications to activate persulfate. However, it should be noted that lime is a solid and would have to be injected as a slurry, whereas liquid alkaline reagents such as sodium hydroxide can be injected in solution. The use of lime has been reserved mainly for activation of potassium persulfate, which is also injected as a slurry due to its low solubility, and sodium hydroxide (25% solution is typical) is commonly matched with the highly soluble sodium persulfate.
Alkaline activation should be considered where there is limited soil buffering capacity of the geologic media in the treatment zone. A laboratory base titration test with a soil-groundwater slurry can be used to measure the amount of base required to raise the pH, including the buffering capacity of the site-specific mineralogy. Persulfate oxidation reactions produce acid, and therefore have the potential to lower the pH as a result of persulfate decomposition. In water, without soil present to buffer the pH, the pH generally drops to the range of 1.5–2.5, depending on the amount of persulfate used. This change in conditions could act to mobilize naturally occurring and/or anthropogenic metals present in the soil and should be monitored closely. The alkaline activation can be dosed appropriately to offset the acid that is formed from persulfate decomposition.
Alkaline-activated persulfate has been shown to be effective at degrading chlorinated ethanes (e.g., 1,1,1-TCA, 1,1-DCA, 1,2-DCA) and methanes (e.g., chloroform) in addition to 1,4-dioxane. The reason for degradation of chlorinated ethanes and methanes appears to be the formation of the superoxide (reducing) radicals in addition to the oxidative radicals when alkaline activation is used (Smith 2020).
Heat activation
Heat activation of persulfate is very effective at initiating the production of sulfate radicals (ITRC 2005). Thermal activation is accomplished by heating the treatment zone to achieve temperatures of 30°C or upward. The higher the treatment zone temperature, the more rapid the reaction kinetics that produce sulfate radicals and degrade contaminants. Subsurface heating can be accomplished using ISTR methods (e.g., thermal resistive heating, thermal conductive heating), through exothermic chemical processes such as hydrogen peroxide decomposition, or with application of heat with an ex situ treatment unit as part of a recirculation delivery method. However, due to higher costs associated with ISTR methods, this heating approach should primarily be considered for sites where recalcitrant chlorinated compounds are co-contaminants that are present alongside with 1,4-dioxane. Many of the VOCs are removed via subsurface heating, thereby decreasing the amount of persulfate required and offsetting the cost of heating.
Hydrogen peroxide is the reagent used with ISCO that is most associated with heat generation, and this activation method is discussed below. Studies have shown that the soil oxidant demand for persulfate can be lower than permanganate (Smith et al. 2018). However, oxidative radicals can also be scavenged by carbonates and chloride ions. While not always an issue even when these ions are present, potential interferences can be evaluated in a bench-scale study. If these scavengers are present at high concentrations, they can reduce oxidant effectiveness.
Use of in situ thermal technology on sites where the recalcitrant chlorinated ethanes and methanes are present, for example, can be followed by a persulfate application, using the synergistic mechanism of thermal degradation of contaminants and thermal activation of persulfate to form sulfate-free radicals. Take care that contaminants do not volatilize into the soil gas and migrate off site before destruction occurs (otherwise, consider use of SVE). Heat activation of persulfate is used less frequently relative to alkaline activation and iron activation.
Hydrogen peroxide activation
Hydrogen peroxide, itself a strong oxidizer, has been used to activate persulfate. However, in the presence of subsurface minerals, these oxidants decompose to form the reactive species described elsewhere in this section (sulfate radical, hydroxyl radicals, superoxide, etc.) (Petri et al. 2011). Because of this matrix demand, hydrogen peroxide is typically added in excess of its stochiometric requirement in the activation of persulfate. Also, the hydrogen peroxide–derived radicals likely play an important role in degradation observed in these systems.
Hydrogen peroxide decomposition also generates heat, and increased temperature from this reaction has been mediated by controlled dosing of hydrogen peroxide, thereby using hydrogen peroxide as achieving heat-activated persulfate conditions (Cronk and Cartwright 2006). Hydrogen peroxide activation of persulfate is used less frequently than alkaline activation or iron activation.
Activation with zero-valent iron
The slow-release characteristic of potassium persulfate can be leveraged to provide for sustained oxidation of 1,4-dioxane in situ. Additionally, persulfate longevity (including either sodium or potassium persulfate) can be extended through controlled activation such as from the slow release of ferrous iron from zero-valent iron (ZVI). Permeable reactive barriers (PRBs) targeting the reduction of 1,4-dioxane from source/high flux areas, or to prevent off-site migration, can be constructed using slow-release persulfate and ZVI such as through slurry injection, in shallow trenches, or through the placement of slow-release “candles” (persulfate and/or permanganate and ZVI in parrafin wax) (Kambhu et al. 2017). The concept of a PRB in which slow-release potassium persulfate activation is instigated by ZVI has recently been evaluated via column treatability studies performed by PeroxyChem (Smith et al. 2018). These studies indicated that ZVI-activated potassium persulfate could decrease 1,4-dioxane concentrations to below detection limits. A case study highlighting persulfate treatment of 1,4-dioxane is included here.
Selection of activation method
As discussed above, alternative persulfate activation chemistries have been evaluated for their effectiveness in degrading 1,4-dioxane. Alkaline conditions, iron, heat, and hydrogen peroxide are reported to be effective in destroying 1,4-dioxane when it is the single contaminant. If, however, 1,4-dioxane is comingled with chlorinated ethanes (e.g., 1,1,1-TCA) or chlorinated methanes, then either alkaline or hydrogen peroxide activation should be considered, as these activation methods generate relatively more hydroxyl radicals and other reactive species, and thus can treat a wider range of organic compounds.
Bench testing—kinetics of 1,4-dioxane degradation using persulfate
Félix-Navarro et al. (Félix-Navarro et al. 2007) studied the kinetics of 1,4-dioxane degradation by persulfate oxidation. Specifically, this bench testing evaluated the influence of temperature, sodium persulfate concentration, and pH on the rate of 1,4-dioxane degradation by persulfate. The objective for the oxidation of 1,4-dioxane by persulfate was to reach the required activation energy (21.0 kilocalories per mole [kcal/mol]) so as to increase the oxidation rate through the generation of highly reactive species such as sulfate radicals (oxidation potential of 2.5 V) and hydroxyl radicals (oxidation potential of 2.8 V). Félix-Navarro et al. (Félix-Navarro et al. 2007) studied the variation of these parameters for their additional effect on the rate of degradation.
Regarding the variation of temperature, testing was conducted in an insulated reaction beaker with an initial 1,4-dioxane concentration of 1.13 millimolar (mM [99.6 mg/L]), and an initial persulfate concentration of 25 mM (6.0 g/L as sodium persulfate). The rate of 1,4-dioxane degradation was evaluated at temperatures of 25°C, 30°C, 40°C, and 50°C. Results indicated that the higher the temperature, the faster the rate of 1,4-dioxane degradation.
Next, testing was performed to evaluate the effects of varied persulfate concentrations. Testing was performed with an initial 1,4-dioxane concentration of 1.13 mM at a constant temperature of 25°C. Persulfate concentrations were varied from 12.5, 25, 50, and 100 mM (3.0 g/L to 24 g/L as sodium persulfate). Results indicated that the higher the initial persulfate concentration, the faster the rate of 1,4-dioxane degradation.
Lastly, testing was performed to evaluate the effects of pH on the rate of 1,4-dioxane degradation. Testing was performed with an initial 1,4-dioxane concentration of 1.13 mM with a persulfate concentration of 25 mM. The pH was varied from 3, 5, 7, 9, to 11. Results indicated that the higher the pH, the slower the rate of 1,4-dioxane degradation. One rationale to explain this result was that the carbon dioxide produced in the higher pH ranges resulted in increased bicarbonate and carbonate concentrations (carbonates are free radical scavengers). Conversely, the faster degradation rates at low pH were hypothesized to result from increased formation of oxonium ions reaction intermediates that facilitate ring opening and oxidation of 1,4-dioxane. Higher pH in persulfate systems favors formation of the hydroxyl radical and can therefore result in a wider range of oxidizing species capable of degrading a wider range of commingled contaminants, as discussed above.
6.5.1.1.2 Sodium and Potassium Permanganate
There are two common forms of permanganate—potassium permanganate (KMnO4) and sodium
permanganate (NaMnO4). Both forms of permanganate are strong oxidizing agents with a unique affinity for oxidizing organic compounds, including chlorinated ethenes (e.g., PCE, TCE). Permanganate also reacts with 1,4-dioxane, but at a slower reaction rate. Both forms of permanganate are available in a range of purities and have similar chemical reactivities. KMnO4 is a crystalline solid from which aqueous solutions up to 4% can be prepared. NaMnO4 is usually supplied as a concentrated liquid (40%) but is usually diluted on site and applied at lower concentrations. Additional background on permanganate is presented in ITRC’s ISCO-2 Guidance Document (ITRC 2005).
Both forms of permanganate dissociate to form MnO4,which has an oxidation potential of 1.7 V. Waldemer and Tratnyek (Waldemer and Tratnyek 2004) determined the kinetics of permanganate oxidation for a number of environmental contaminants and found that the second-order rate constant for the reaction of permanganate and 1,4-dioxane was four orders of magnitude lower than that found for permanganate and TCE. The relative stability of permanganate in the subsurface [e.g., on the order of months; (ITRC 2005)] could be beneficial for the oxidation of 1,4-dioxane in the event that chlorinated ethenes such as TCE are more quickly degraded. For degradation of 1,4-dioxane by permanganate to be effective, permanganate concentrations must be relatively high, and permanganate must persist at elevated concentrations for a prolonged period of time. A low natural oxidant demand (Inoue et al.) will further improve the permanganate’s longevity.
A treatability study may be conducted to determine site-specific effectiveness and predict the remediation time frame. The viability of applying permanganate for 1,4-dioxane remediation should be determined on a case-by-case basis and depends on the remedial strategy and treatment goals.
An ESTCP project was performed that evaluated the effectiveness of permanganate for the oxidation of 1,4-dioxane (Evans et al. 2018). Specifically, this study evaluated the use of slow-release potassium permanganate from chemical oxidant cylinders (chemical oxidant embedded in a slow-release wax) for application in the treatment of dissolved plumes of 1,4-dioxane either as a sole contaminant or comingled with chlorinated ethenesand ethanes. Initial batch testing in deionized water revealed a linear rate of decrease in the initial 1,4-dioxane concentration (10,000 µg/L) with increased permanganate concentration over the range of 0.066%–1.329% permanganate solutions. From these results, a second-order rate constant of 4.3 x 10-5 M-1s-1 was estimated for use in planning field pilot testing.
Additional batch testing was performed to evaluate permanganate’s effectiveness for the oxidation of 1,4-dioxane in soil and groundwater from a test site (Naval Air Station North Island, San Diego, California); and comingled with TCE; cis-1,2-DCE; 1,1-DCE; and 1,1-DCA. Results indicated a similar order of magnitude second-order rate constant of 3.4 x 10-5 M-1s-1. The results further indicated that permanganate achieved an approximate 90% reduction in 1,4-dioxane concentrations in the initial approximately 350 hours of testing. Moreover, results indicated that chlorinated ethene concentrations were reduced by over 99% within the first 2 hours of reaction and that the presence of these chlorinated compounds did not affect the rate of 1,4-dioxane oxidation. Chlorinated ethanes were not degraded by permanganate.
6.5.1.1.3 Ozone
Ozone with an oxidation potential of 2.07 V is a powerful oxidizer to be considered for its effectiveness in degrading 1,4-dioxane. In situ applications for ozone include ozone sparging, or the ozonation of water that is subsequently injected. Like treatment of other organics, ozone oxidation of 1,4-dioxane may occur due to direct reaction with ozone or may occur indirectly through reactions with hydroxyl radicals generated as the result of the interaction of ozone with water or ozone and minerals in the soil matrix.
Hydroxyl radicals with an oxidation potential of 2.8 V are an even more powerful oxidizer that can destroy 1,4-dioxane. Given the recalcitrance of 1,4-dioxane, most field applications have targeted the increased generation of hydroxyl radicals that results from the combination of ozone and hydrogen peroxide (i.e., peroxone), which is discussed further in Section 6.5.1.1.4. Previous ITRC documentation (ITRC 2005) offers details on the general chemistry and application of ozone and for ozone with hydrogen peroxide.
6.5.1.1.4 Ozone/Hydrogen Peroxide
The combination of ozone and hydrogen peroxide is known in chemistry as peroxone and has been used for many years to treat contaminants in water ex situ (see Section 6.2.4.1). Combined ozone and hydrogen peroxide have also been used for specific contaminant remediation applications, such as TCE and explosives, and has additionally been evaluated for application to the in situ degradation of 1,4-dioxane.
The success of in situ application of ozone/peroxide is challenging and depends on the effective contact between ozone and hydrogen peroxide in groundwater to form hydroxyl radicals. Numerous techniques are available for the in situ delivery of ozone and hydrogen peroxide into groundwater. Specifically constructed wells or sparging systems may be required for effective application of these combined oxidants for the in situ treatment of 1,4-dioxane in groundwater. An introduction to the aqueous chemistry of combined ozone and hydrogen peroxide can be found in previous ITRC guidance (ITRC 2005).
Both ozone and combined ozone with hydrogen peroxide have been evaluated for their effectiveness in degrading 1,4-dioxane in groundwater. A 2006 bench study was performed in support of the Cooper Drum Superfund site in South Gate, Los Angeles County, California (Schreir et al. 2006). In this bench study, the effectiveness of combining ozone with hydrogen peroxide was evaluated with the objective of degrading 1,4-dioxane through the increased generation of hydroxyl radicals that result from combining these two reagents. Initial testing revealed that ozone alone (940 mg dose) resulted in identical 1,4-dioxane degradation as resulted from combined ozone and hydrogen peroxide. In these initial tests, 1,4-dioxane concentrations were reduced in site groundwater from 680 µg/L to less than 3 µg/L for (1) ozone alone, (2) ozone plus low-dose hydrogen peroxide (0.07%), (3) ozone plus high-dose hydrogen peroxide (0.35%), and (4) a higher dose of ozone alone (1,100 mg).
Based on the results, further testing was performed to evaluate if the geochemistry of the site groundwater was affecting the initial test results. For this next round of testing, deionized (DI) water was spiked with 1,4-dioxane, with some reactors receiving additional enhancements (e.g., ferrous iron and chelated iron) to simulate concentrations found in site groundwater. These tests did not include hydrogen peroxide. Results revealed that ozone alone (in DI water) achieved only 75% removal of 1,4-dioxane (400 µg/L to 98 µg/L). However, in reactors that received ferrous iron or chelated iron, results were comparable with the initial testing, wherein complete destruction of 1,4-dioxane was reported (400 µg/L to less than 3 µg/L). Based on these results, it was hypothesized that iron naturally present in site groundwater was enhancing the ozone’s effectiveness. That ozone can react with naturally occurring groundwater constituents such as iron and manganese to generate hydroxyl radicals had been demonstrated previously (Bower and Miller 2002).
Lastly, results from the 2006 bench study indicated that some of the adverse water quality impacts resulting from the use of ozone alone are eliminated or lessened through the application of ozone combined with hydrogen peroxide. Bromate, total and hexavalent chromium, and copper were reported at higher concentrations associated with the injection of ozone alone versus testing with combined ozone and hydrogen peroxide. One notable variation was for manganese, which was reported at a higher concentration following application of combined ozone and hydrogen peroxide as compared with ozone alone.
These bench study results highlight the need for bench testing to better understand the site chemistry prior to performing field pilot tests or full-scale ISCO for 1,4-dioxane. One objective for bench testing should be to evaluate the need for hydrogen peroxide addition in combination with ozone and whether naturally occurring soil and groundwater constituents may similarly enhance the effectiveness of ozone.
In December 2006, a pilot study was conducted at the Cooper Drum Superfund site to determine whether ozone, with or without hydrogen peroxide, can destroy 1,4-dioxane and comingled chlorinated VOCs. The study involved the use of specially constructed wells with an ozone diffuser in the bottom of the injection well and a hydrogen peroxide diffuser higher in the well, such that the two chemicals interact in the subsurface to form the hydroxyl radical. Initial injections were of ozone alone, which resulted in significant reductions in 1,4-dioxane and TCE concentrations. During that time, 1,4-dioxane concentration was reduced from 750 µg/L to 47 µg/L. Five months later, hydrogen peroxide injections were initiated, and it was determined that optimal results were achieved with the combination of 16% hydrogen peroxide and 0.9 kg ozone per day (2 lb ozone per day) per injection well. Reduction as high as 88% was achieved in some of the monitoring wells, and all target contaminant concentrations decreased during the pilot-test period (Sadeghi et al. 2006). The pilot test was considered successful, and an initial decision was made to use a combined ozone/hydrogen peroxide approach for full-scale treatment; however, the implementation did not occur for other reasons.
6.5.1.1.5 Catalyzed Hydrogen Peroxide (aka Modified Fenton’s Reagent)
Catalyzed hydrogen peroxide (CHP, also known as the Modified Fenton’s Reagent [MFR] process) consists of activation of hydrogen peroxide with a transition metal. Ferrous iron (Fe[II]) is the most common activator. Hydrogen peroxide is very unstable in the subsurface; thus, transport of hydrogen peroxide away from injection points is difficult to achieve, although stabilizers have reportedly been used to increase hydrogen peroxide distribution. Ferrous iron is also converted to less-soluble ferric iron (Fe[III]), which can precipitate out of solution in oxidizing conditions and pH >3. Chelating agents can be used to increase iron solubility at higher pH. Hydrogen peroxide decomposition releases heat and oxygen gas, both of which can contribute to vigorous reactions in the subsurface.
CHP reactions produce a wide range of reactive radical species, including the hydroxyl radical (OH•), superoxide (O2•–), hydroperoxide anions (HO2 –), and others. These reactive species can treat a wide range of organic compounds, including 1,4-dioxane and other organic contaminants. Additional information on CHP can be found in ITRC (2005) and other resources.
CHP has been applied as full-scale technology for treatment of a comingled plume that included 1,4-dioxane at a former drum conditioning Superfund site in Kingston, New Hampshire. Three separate treatment areas—(Area A, Area B, and Area C)—were designated for treatment. Primary COCs at the site include BTEX, PCE, TCE, 1,1‐DCA, and 1,4-dioxane. The ISCO remedy chosen for the site consisted of sodium hydroxide–activated sodium persulfate as the primary oxidant and CHP as a secondary oxidant. Historical levels of 1,4-dioxane were as high as 200 µg/L in Area B and 32 µg/L in Area C. Remediation was implemented in three phases. During Phase III, a total of 22,990 gallons of CHP was injected into 34 wells in one treatment area, and 3,400 gallons of CHP was injected into 24 wells in another treatment area. Following Phase III of the treatment program, most of the ISCO target treatment areas were below site cleanup levels for a majority of primary COCs, with concentrations in the remaining areas in an acceptable range to transition to MNA [ISOTEC Case Study No. 67; (Dombrowski et al. 2010)].
6.5.1.2 Phytoremediation
Phytoremediation is the use of plants to treat environmental impacts. In general, phytoremediation may consist of plant-mediated degradation, extraction, volatilization, or stabilization. More information about phytoremediation can be found in the ITRC Phytotechnology Technical and Regulatory Guidance and Decision Trees, Revised (ITRC 2009). These treatment reactions may be mediated by the plants directly or through reactions with microbial or fungal communities that live within the root zone (rhizodegradation) or within the plants themselves (endophytes).
Phyto-extraction is the most common and well-understood method of phytoremediation for treatment of 1,4-dioxane. This method works because as plants transpire (i.e., take in) groundwater, they also pull in 1,4-dioxane present in the groundwater. Being miscible in water and nonsorptive, the 1,4-dioxane moves up the plant and is transpired out of the leaves. Once released into the atmosphere, the 1,4-dioxane is photodegraded by UV light from the sun (Aitchison et al. 2000).
Other mechanisms may also result in degradation of 1,4-dioxane in interactions with plants. For example, bacteria that have been shown to degrade 1,4-dioxane in groundwater systems may also have the potential to live in symbiotic communities near or within plants. Microorganisms capable of cometabolic biodegradation in the rhizosphere have been investigated as having the potential to degrade 1,4-dioxane in a phytoremediation system (Sun 2010).
Phytoremediation design consists of selecting the type of system and the location at which it should be placed. Because phytoremediation of 1,4-dioxane typically consists of phyto-extraction, water-intensive plants such as hybrid poplars are typically selected. Assuming the phytoremediation system will consist of a number of trees, the next design parameters are how many to plant and where to place them. Tree spacing guidance is available in the ITRC Phytotechnology Technical and Regulatory Guidance and Decision Trees, Revised. Tree spacing and the number of trees should also consider groundwater flux. Trees may be thought of as small pumping wells.
Phytoremediation systems consisting of many units have been shown to create a cone of depression (Gestler 2016); therefore, the volume of groundwater transpired by each tree and the number of trees should be compared against the groundwater flux through the area. Additionally, trees transpire relatively more water as they mature, so tree spacing will depend on how many years of growth can occur before the design groundwater removal is met.
For faster time frames (2–3 years), relatively more trees may be required. Another hydraulic parameter to consider is the groundwater velocity relative to the length of the tree stand. Because trees are dormant in the winter in some areas, relatively little groundwater extraction by trees occurs in the winter months. To avoid 1,4-dioxane moving all the way past the phytoremediation plot, the length of the plot parallel to the direction of groundwater flow is typically longer than the travel time during the trees’ dormant period.
Another consideration is the depth to which trees will be planted. Ideally, trees will be planted such that they transpire primarily 1,4-dioxane-impacted groundwater. When 1,4-dioxane is present near the top of the water table, this is relatively straightforward. Trees should be planted so that they engage groundwater, do not transpire shallow soil moisture, and require little to no irrigation once they are established. Newly planted trees may require more irrigation and other maintenance to ensure survival the first few seasons after planting.
Use of tree poles (long straight branchless trees that are harvested elsewhere and re-planted at the site) is a typical way to engage groundwater at depth. If 1,4-dioxane is stratified vertically in groundwater and is not in the shallowest portion of the water-bearing zone, specialty installations (e.g., the TreeWell® system) can be used to install tree rooting zones in the depth of 1,4-dioxane impacts while isolating the tree from unimpacted shallower groundwater.
One last consideration is that the 1,4-dioxane may be present along with other co-contaminants that may be more toxic to plants than the 1,4-dioxane itself. For example, chlorinated solvents at high concentrations can be toxic to plants. Additionally, inorganic compounds such as metals or highly saline groundwater can be toxic to plants. If toxicity of co-contaminants is a concern at a specific field site, a pilot study may be conducted to evaluate the survival of trees under site-specific conditions. A case study highlighting phytoremediation of 1,4-dioxane is included here.
6.5.2 Emerging Options for Groundwater (In Situ)
Emerging options are technologies that may be partially demonstrated or researched and may include technologies implemented under laboratory bench-scale or pilot-scale situations. Typically, less documentation, research, or validation is available.
6.5.2.1 Monitored Natural Attenuation
MNA is a remediation technology in which natural processes are used to achieve site-specific objectives. While MNA does not involve active treatment, it includes periodic groundwater monitoring and comparison of results to performance expectations and is therefore not a “do-nothing” approach. MNA is often performed in conjunction with other technologies as part of a treatment train, or it can be considered as an alternative approach for sites where traditional in situ treatment technologies cannot be employed. MNA is typically used in downgradient, lower concentration areas or as a polishing technology in source areas after more aggressive technologies have been implemented. MNA may not be appropriate under some site-specific conditions, such as when 1,4-dioxane concentrations pose unacceptable risk to receptors or when attenuation time frames are longer than is acceptable to stakeholders. In addition to monitoring concentrations of target compounds, MNA programs generally include monitoring of geochemical conditions to assess the favorability of attenuation of target compounds under site-specific conditions as part of a multiple-lines-of-evidence approach. An ESTCP project ER-201730 is in progress (in 2020) to develop a framework to evaluate natural attenuation of 1,4-dioxane.
MNA is based on attenuation caused by one or more of the following processes: biodegradation; dispersion; dilution; sorption; volatilization; radioactive decay; and chemical or biological stabilization, transformation, or destruction of contaminants [(USEPA 1999b); (ITRC 1999)]. For organic compounds such as 1,4-dioxane, processes that degrade or destroy target compounds are preferred by USEPA and other regulatory agencies.
Several of 1,4-dioxane’s chemical properties affect the degree to which this compound may be treated with MNA. 1,4-Dioxane’s relatively low sorption potential makes it more likely to become reduced in concentration due to dilution or dispersion, but also more likely to travel to downgradient areas due to lesser retardation than other compounds. 1,4-Dioxane’s miscibility in water and low Henry’s law constant make it unlikely to volatilize into soil gas.
Biological processes that can potentially degrade 1,4-dioxane in MNA are the same as those discussed in the context of its fate and transport (see Section 3.1.7) as well as those used in engineered systems (see Section 6.5.2.2 [aerobic cometabolic biodegradation] and Section 6.5.2.3 [aerobic metabolic biodegradation] for more details). Biological degradation may occur under aerobic conditions. Microorganisms capable of cometabolic biodegradation appear to be widespread, and fewer microorganisms have been identified that can metabolize 1,4-dioxane and use it as a growth-supporting substrate. Anaerobic biological processes that degrade 1,4-dioxane are not currently thought to be relevant to MNA, although ethane, and possibly methane, generated under anaerobic conditions may serve as co-substrates for cometabolic degradation in aerobic zones.
Chlorinated solvents and metals have been found to be inhibitory to 1,4-dioxane degradation under some conditions, though some of these studies were performed with solvent and metals concentrations much higher than those typically observed at field sites [(Adamson et al. 2015); (Pornwongthong et al. 2014); (Zhang, Gedalanga, and Mahendra 2016)]. 1,4-Dioxane degradation after oxygen addition to solutions containing reduced iron has been observed in laboratory studies, suggesting that Fenton’s reaction processes may result in some abiotic degradation of 1,4-dioxane in ambient conditions (Barajas-Rodriguez 2016), though other research indicates bacteria may mediate this reaction by generating hydrogen peroxide (Sekar and DiChristina 2014).
From a big-picture perspective, MNA of 1,4-dioxane in groundwater appears to be an ongoing process at some sites impacted by this compound. (Adamson et al. 2015) reviewed concentration data over time for 1,4-dioxane and several common chlorinated compounds at multiple sites. They found that concentrations of 1,4-dioxane were decreasing over time at a statistically significant level at an appreciable number of sites, suggesting that natural attenuation was occurring at those sites. They also found that apparent degradation rates of 1,4-dioxane were similar to those of TCE and 1,1-DCE, and that attenuation rates were positively correlated with dissolved oxygen and negatively correlated with dissolved metals and chlorinated solvent concentrations.
One explanation for the observed attenuation and this positive correlation is aerobic biological degradation via either growth-linked metabolic or cometabolic processes. Recent studies showing that soluble di-iron monooxygenase enzymes capable of oxidizing 1,4-dioxane are prevalent in groundwater at many sites further support this hypothesis [(Gedalanga et al. 2014); (Gedalanga et al. 2014); (Li, Deng, and Li 2019); (Chiang et al. 2012)]. Given the low 1,4-dioxane concentrations at many sites (low µg/L concentrations) and the relatively high Ks for growth-linked degradation by known strains [e.g., 160 mg/L for Pseudonocardia dioxanivorans CB1190; (Mahendra and Alvarez-Cohen 2006)], cometabolism may be the most plausible explanation for attenuation at several of these sites (see Sections 6.5.2.2).
Of particular interest is cometabolism can be supported by 1) naturally occurring primary substrates in groundwater; 2) co-contaminants such as THF (which induces enzymes that catalyze 1,4-dioxane degradation); 3) by-products, such as ethane, formed during reductive dechlorination of CVOCs (often present at 1,4-dioxane sites and treated via organic substrate addition); or 4) other primary substrates that may be present. As previously noted, however, methanotrophic bacteria’s ability to biodegrade 1,4-dioxane, and subsequently, the potential for attenuation of 1,4-dioxane linked to bacterial methane oxidation in groundwater, is presently uncertain as both positive and negative data have been reported [(Mahendra and Alvarez-Cohen 2006); (Hatzinger et al. 2017)].
Oxygen flux into the system may be from upgradient groundwater flow or recharge from above. Aerobic conditions may be present at transitional areas downgradient of anaerobic source zones. A specific dissolved oxygen (DO) threshold above which there is sufficient oxygen for 1,4-dioxane biodegradation has not yet been established. However, studies on engineered cometabolic biodegradation systems targeted a DO concentration of at least 4 mg/L (Lippincott et al. 2015) and modeled a lower limit of 1.5 mg/L (Barajas-Rodriguez and Freedman 2018). Additionally, research into aerobic biodegradation of VC has found that trace concentrations of DO (0.02 mg/L) can be sufficient for aerobic processes to occur (Gossett 2010).
Design of MNA programs for 1,4-dioxane should consider the following:
- If chlorinated solvents or metals impacts are known to be present as co-contaminants at the site, the concentrations of these co-contaminants should be compared against those observed to have inhibitory effects. In previous studies, 1,1-DCE had a moderate effect on the kinetics of 1,4-dioxane biodegradation by Pseudonocardia dioxanivorans CB1190 at 0.5 mg/L, and was inhibitory at 5 mg/L. cis-1,2-DCE reduced degradation rates at a concentration of 5 mg/L, whereas TCE and 1,1,1-TCA did not have a significant effect on degradation rates of 1,4-dioxane by Pseudonocardia dioxanivorans CB1190 at this same concentration. TCE but not 1,1,1-TCA showed toxicity at 50 mg/L (Zhang, Gedalanga, and Mahendra 2016). For metals, copper was observed to have an inhibitory effect on 1,4-dioxane biodegradation by Pseudonocardia dioxanivorans CB1190 at a concentration of approximately 1 mg/L, while other metals tested (including cadmium, nickel, and zinc) did not have an effect until concentrations of 10 mg/L (Pornwongthong et al. 2014). It is not currently known whether other microorganisms are similarly affected by these chlorinated compounds and metals.
- 1,4-Dioxane analytical methods should be selected to achieve project-specific RLs (see Section 4 for additional details).
- Geochemical data and/or field parameters should be collected to evaluate site conditions relative to the aerobic conditions optimal for 1,4-dioxane biodegradation.
- Monitoring of co-substrates that can potentially drive cometabolic biodegradation may provide value. Though each site is unique, ethane, ethene, and THF appear to stimulate cometabolic degradation of 1,4-dioxane to a greater extent than methane.
- qPCR can be used to quantify a variety of genes associated with metabolic and cometabolic degradation of 1,4-dioxane. Detection of these genes at elevated levels could provide a line of evidence indicating the presence and activity of bacteria associated with biodegradation of 1,4-dioxane. Readers should note that different service providers call qPCR targets by different names.
- CSIA can be used to assess the degree of isotopic enrichment along groundwater flow paths or over time. These results can be used in conjunction with literature enrichment factors to indicate 1,4-dioxane degradation. Because relatively low enrichment factors for carbon have been reported for 1,4-dioxane (Bennett et al. 2018), use of both carbon and hydrogen CSIA (i.e., 2D CSIA) may provide more resolution.
Some progress has been made on the development and application of molecular and environmental diagnostic tools to document MNA of 1,4-dioxane in groundwater [(Chiang et al. 2012); (Chiang et al. 2016); (Gedalanga et al. 2014); (Bennett et al. 2018)]. These tools currently include the application of qPCR to detect and quantify specific enzymes (both metabolic and cometabolic) that have been reported to catalyze 1,4-dioxane degradation in bacteria and the development of a CSIA technique to quantify both δ13C and δD in 1,4-dioxane. See the ITRC Environmental Molecular Diagnostic Guidance Document for more detail on qPCR and CSIA.
A current limitation of molecular tools is that the presence of metabolic or cometabolic genes in groundwater doesn’t necessarily correlate to the occurrence or rate of 1,4-dioxane transformation. CSIA can overcome this limitation by providing evidence of isotopic enrichment, which is a clear indication of 1,4-dioxane degradation (biotic or potentially abiotic), but additional studies are required to validate this approach in field samples and to further document isotope fractionation factors for 1,4-dioxane by different mechanisms. In the future, it may be possible to develop a framework that includes modeling of degradation rates, geochemical data, molecular tools, and CSIA as a framework for documenting the MNA of 1,4-dioxane in groundwater, much as has been done for chlorinated solvents over the past few decades.
The scientific understanding of 1,4-dioxane attenuation is continuing to evolve as the result of ongoing research and field application. In the future, additional microorganisms or biological processes that degrade 1,4-dioxane may be discovered. Also, further understanding of abiotic processes that result in 1,4-dioxane degradation (e.g., iron-mediated reactions, or reactions between 1,4-dioxane and mineral surfaces) may provide additional natural attenuation mechanisms.
6.5.2.2 Aerobic Cometabolic Biodegradation
Aerobic microorganisms can biodegrade 1,4-dioxane through two physiologically distinct processes (Figure 6-2). In some cases, microorganisms metabolize 1,4-dioxane as a sole source of carbon and energy. This process can result in mineralization of 1,4-dioxane to carbon dioxide and increases in the number (growth) of the 1,4-dioxane-degrading microorganisms. In other cases, microorganisms can fortuitously degrade 1,4-dioxane through cometabolism. Cometabolism occurs with many recalcitrant compounds but often does not provide active microorganisms with sufficient carbon and energy to support growth, as the contaminant undergoes only limited and/or slow degradation. Cometabolic processes therefore usually require a primary substrate (e.g., propane, toluene) that not only supports the growth of the active microorganisms but also leads to expression of the appropriate nonspecific contaminant-degrading enzymes by these microorganisms. From a bioremediation perspective, a key difference between 1,4-dioxane metabolism and cometabolism is the concentration of 1,4-dioxane at which these processes are effective. Due to the energy needed to maintain the activity and viability of 1,4-dioxane-metabolizing microorganisms, metabolic 1,4-dioxane degradation is limited to relatively high concentrations of 1,4-dioxane (≥250 µg/L) (Barajas-Rodriguez and Freedman 2018). By contrast, cometabolism can treat both high and low (<100 µg/L) concentrations of 1,4-dioxane and is therefore likely to be the most effective biological approach for remediating the majority of the environmentally relevant concentrations of 1,4-dioxane (≤100 µg/L) (Barajas-Rodriguez and Freedman 2018). Because the monooxygenase enzymes that enable microorganisms to cometabolically degrade 1,4-dioxane are typically highly nonspecific monooxygenase enzymes, cometabolic biotreatment process can also concurrently degrade 1,4-dioxane and some of its common chlorinated co-contaminants (Rasmussen et al. 2020).
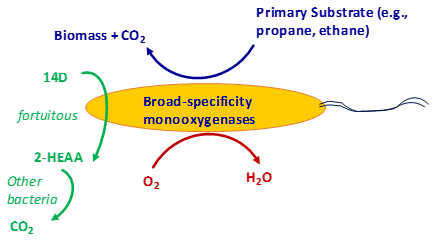
Figure 6-2. General concept cometabolism of 1,4-dioxane.
Source: ITRC 1,4-Dioxane Team, 2020.
There are numerous examples of microorganisms that do not grow on 1,4-dioxane as a sole source of carbon and energy but can cometabolically degrade this compound after growth on other substrates. For example, microorganisms that can grow on THF, a close structural analog of 1,4-dioxane, have often been reported to cometabolically degrade 1,4-dioxane. These microorganisms include several bacterial genera [e.g., Rhodococcus (Bock, Kroppenstedt, and Diekmann 1996); (Sei et al. 2013) and Pseudonocardia [(Vainberg et al. 2006); (Mahendra and Alvarez-Cohen 2006); (Bennett et al. 2018)] and fungi (Skinner, Ciuffetti, and Hyman 2009)]. Note, however, that not all members of these specific genera degrade 1,4-dioxane.
Other primary substrates reported to support cometabolic degradation of high concentrations of 1,4-dioxane (≤500 mg/L) include toluene and methane (Mahendra and Alvarez-Cohen 2006); however, methane-dependent degradation of 1,4-dioxane has not been observed in other studies of methane-oxidizing bacteria or with highly purified methane monooxygenase (Hatzinger et al. 2017). Bacteria grown on non-methane alkanes—including ethane (Hatzinger et al. 2017), propane [(Burback and Perry 1993); (Mahendra and Alvarez-Cohen 2006); (Lan, Smith, and Hyman 2013)], n-butane, n-pentane, isobutane, and isopentane (Lan, Smith, and Hyman 2013)—also cometabolically degrade 1,4-dioxane. Genome-based studies have recently shown that many gaseous alkane-oxidizing bacteria possess multiple monooxygenases that can potentially oxidize alkanes or 1,4-dioxane [(Cappelletti et al. 2015); (Shields-Menard et al. 2014); (Deng et al. 2019); (Tupa and Masuda 2018)]. Consequently, the enzymes responsible for oxidation of 1,4-dioxane have not been unequivocally identified in many cometabolically active alkane-oxidizing bacteria.
A recent 13C- and 2H-based CSIA of 1,4-dioxane cometabolism also suggests that different growth substrates may promote the expression of different 1,4-dioxane-degrading enzymes within the same microorganism (Bennett et al. 2018). Despite these uncertainties, recent pure culture studies have shown that some strains of alkane-utilizing bacteria can cometabolically degrade 1,4-dioxane to very low concentrations (≤0.4 µg/L) after growth on gaseous alkanes (Rolston, Hyman, and Semprini 2019). In some cases, these microorganisms can also oxidize some common chlorinated co-contaminants that are frequently found with 1,4-dioxane in ground water (Rasmussen et al. 2020) and can inhibit 1,4-dioxane degradation.
Chlorinated ethenes would be expected to cause toxicity to 1,4-dioxane cometabolizing strains at high concentrations due to the initial formation of epoxides by relevant monooxygenases [e.g., (Mahendra, Grostern, and Alvarez-Cohen 2013); (Hand, Wang, and Chu 2015)]. Note, however, that the concentration at which toxicity occurs and the effect on 1,4-dioxane cometabolism is expected to be specific to both the strain and the cell concentration. The influence of metals on 1,4-dioxane cometabolizing strains is less well-studied than for metabolic strains (see Section 6.5.2.3), although one recent study showed little toxicity of Cr(VI) to two strains at concentrations as high as 10 mg/L (Johnson et al. 2020).
Cometabolic treatment in groundwater typically entails the addition of a primary growth substrate (e.g., an alkane gas such as propane, ethane, or methane) with or without inorganic nutrients or bioaugmentation. This approach is mature, cost-effective, and can be safely applied in situ using several different configurations based on site conditions, including biosparging [e.g., (Lippincott et al. 2015); (Bell et al. 2016); (Horst et al. 2019); (Hatzinger and Lippincott 2019)]; groundwater recirculation with active substrate addition [(Hatzinger et al. 2018); (Chu et al. 2018)]; and passive substrate addition in groundwater wells (Begley et al. 2012). Figure 6-3 provides generalized examples of the two most common in situ treatment designs (biosparging and groundwater recirculation). Both approaches have been successfully demonstrated in the field for treatment of 1,4-dioxane, as described later in this section [(Lippincott et al. 2015); (Chu et al. 2018)]. Cometabolic treatment has several advantages. First, the technology is well-suited for dilute plumes, as are often observed for 1,4-dioxane, because the cometabolic organisms are not required to grow on the contaminant of concern, but rather utilize the substrate that is supplied to the subsurface. Second, very low treatment levels (e.g., low nanogram per liter [ng/L] concentrations) can be achieved for some pollutants (e.g., Hatzinger et al. 2017, 2018). Third, the potential exists to treat multiple contaminants, such as 1,4-dioxane and various chlorinated solvents [(Lippincott et al. 2015); (Chu et al. 2018)] simultaneously, although multiple substrates may be required in some cases (Laurance 2019).
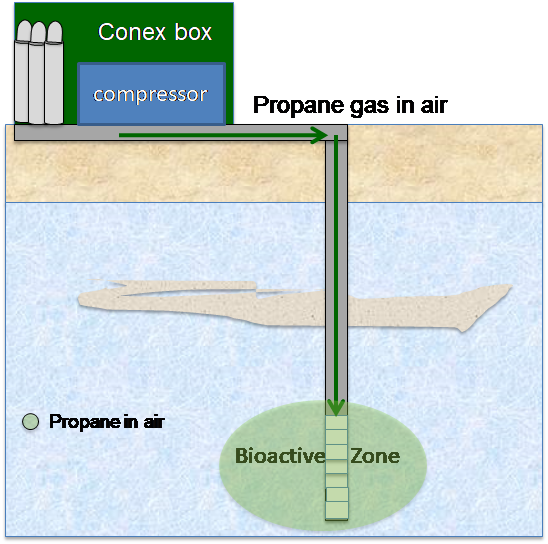
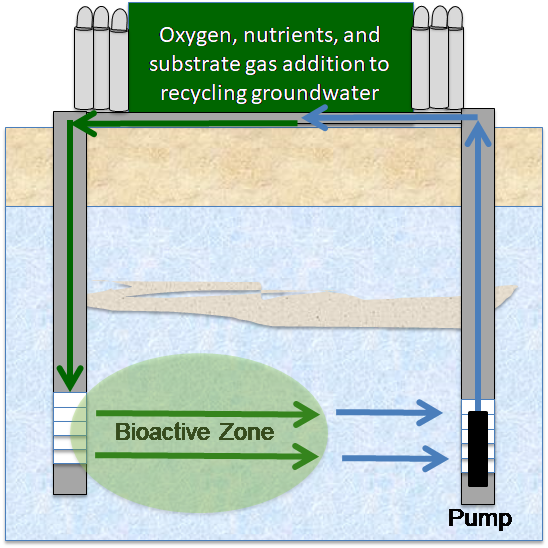
Figure 6-3. Graphic examples of two types of system design used for in situ aerobic cometabolic treatment.
Source: ITRC 1,4-Dioxane Team, 2020.
Cometabolic treatment of pollutants in situ, however, also has some potential limitations. For example, although propane- or ethane-oxidizing bacteria appear widespread in nature, they are not necessarily ubiquitous, so bioaugmentation may be required (Lippincott et al. 2015). However, similar to metabolic 1,4-dioxane degraders (e.g., Pseudonocardia dioxanivorans CB1190), many propanotrophs are actinomycetes that form clumps and filaments when grown in culture. These organisms, such as Rhodococcus ruber ENV425, have also been observed to attach to surfaces in flow-through environments rather than remaining suspended in solution [(Webster, Condee, and Hatzinger 2013); (Hatzinger et al. 2011); (Hatzinger, Lewis, and Webster 2017)]. While biofilm formation may be advantageous for long-term in situ treatment, initial distribution of such bioaugmentation cultures in the subsurface may be a challenge, and the numbers of such organisms detected in groundwater samples may dramatically underestimate the true subsurface population, as most cells may be attached to solids. Also, both cometabolic and metabolic biodegradation of 1,4-dioxane could be affected by the presence of co-contaminants like 1,1,1-TCA and 1,1-DCE, which are present at many 1,4-dioxane-contaminated sites (Mahendra, Grostern, and Alvarez-Cohen 2013).
Cometabolic treatment of 1,4-dioxane has been successfully demonstrated in several field demonstrations. In a field study conducted at Vandenberg Air Force Base in California (Lippincott et al. 2015), propane was added to 1,4-dioxane-contaminated groundwater via biosparging, along with inorganic nutrients and the propanotroph Rhodococcus ruber ENV425. Over the course of the 8-month demonstration, 1,4-dioxane concentrations in the wells receiving propane declined from as high as 1,090 µg/L to between 2 µg/L (the practical quanititation limit) and 7.4 µg/L, representing a 95% to >99% reduction from the starting concentrations. Similar losses were not observed in a control well that did not receive propane. A variety of chlorinated solvents present at low concentrations (~20 to 400 µg/L) were also reduced to <2 µg/L in the treatment wells, but not the control well. A full description of this case study is included here.
In another field study, which was conducted at McClellan Air Force Base in California (Chu et al. 2018) over a 9-month test period, recirculated groundwater was amended with propane and oxygen to stimulate cometabolic biodegradation of 1,4-dioxane (~60 µg/L) and two chlorinated solvents (TCE and 1,2-DCA). Bioaugmentation was not performed. The indigenous microbial population consistently degraded 1,4-dioxane to below 3 μg/L, while the co‐contaminants TCE and 1,2‐DCA (with initial concentrations of ~10 μg/L and 1.4 μg/L, respectively) were decreased to below 1 μg/L and 0.18 μg/L, respectively. A stable treatment efficiency of more than 95% removal for 1,4-dioxane and 1,2‐DCA and more than 90% removal for TCE was achieved in the recirculating cell. Interestingly, the high treatment efficiencies were sustained even without propane and oxygen addition over a 2‐week period, showing this design’s resiliency.
The promising results of these field demonstrations indicates that this remdial technology is quickly evolving from an emerging option to a fully demonstrated approach. Consult the most recent literature when considering this treatment technology.
6.5.2.3 Aerobic Metabolic Biodegradation
Aerobic microorganisms can biodegrade 1,4-dioxane through two physiologically distinct processes (Figure 6-4). In some cases, microorganisms metabolize 1,4-dioxane as a sole source of carbon and energy. This process can result in mineralization of 1,4-dioxane to carbon dioxide and increase the number (growth) of the 1,4-dioxane-degrading microorganisms. In other cases, microorganisms can fortuitously degrade 1,4-dioxane through a process called cometabolism. From a bioremediation perspective, a key difference between 1,4-dioxane metabolism and cometabolism is the concentration of 1,4-dioxane at which these processes are effective. Due to the energy needed to maintain the activity and viability of 1,4-dioxane-metabolizing microorganisms, metabolic 1,4-dioxane degradation is limited to relatively high concentrations of 1,4-dioxane (≥250 µg/L) (Barajas-Rodriguez and Freedman 2018). In contrast, cometabolism can treat both high and low (<100 µg/L) concentrations of 1,4-dioxane and is therefore likely to be the most effective biological approach for remediating the majority of the environmentally relevant concentrations of 1,4-dioxane (≤100 µg/L) (Barajas-Rodriguez and Freedman 2018).
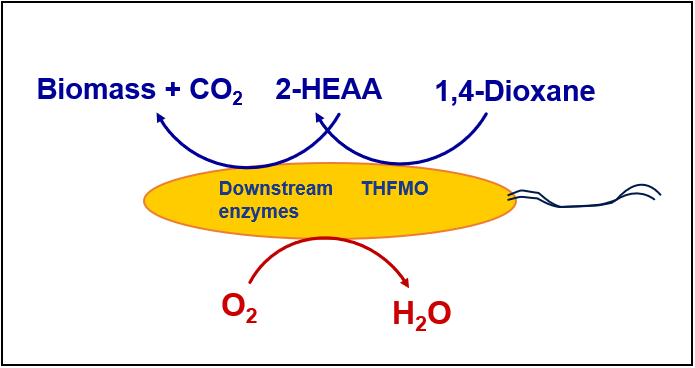
Figure 6-4. General concept of metabolism of 1,4-dioxane.
Source: ITRC 1,4-Dioxane Team, 2020.
Several diverse microorganisms, including fungi (Nakamiya et al. 2005), bacteria [(Parales et al. 1994); (Mahendra and Alvarez-Cohen 2005); (Kim et al. 2009); (Huang et al. 2014); (He et al. 2017)], and microbial consortia (Tusher et al. 2020) can metabolize and grow on 1,4-dioxane. These microorganisms typically grow slowly (long generation/doubling time) and inefficiently (low biomass yield) on 1,4-dioxane. The best-characterized 1,4-dioxane-metabolizing strain is Pseudonocardia dioxanivorans CB1190. This bacterium was originally isolated from a mixed culture of THF-metabolizing bacteria and likely adapted to grow on 1,4-dioxane through mutation (Parales et al. 1994).
The bacterium grows slowly (30-hour doubling time) and inefficiently (<0.1 g biomass per g 1,4-dioxane consumed) on 1,4-dioxane (Parales et al. 1994). The monooxygenase that initiates 1,4-dioxane degradation in Pseudonocardia dioxanivorans CB1190 is tetrahydrofuran monooxygenase (THFMO) (Gedalanga et al. 2014). This enzyme is also thought to initiate 1,4-dioxane degradation in several other 1,4-dioxane-metabolizing and cometabolizing bacteria [(Inoue et al. 2016); (Masuda et al. 2012)]. In some studies, THFMO has also been referred to as 1,4-dioxane monooxygenase and abbreviated as DXMO. This alternative name (and abbreviation) is unnecessary and intrinsically misleading because it suggests, incorrectly, that all microorganisms that express THFMO/DXMO can metabolize and grow on 1,4-dioxane.
Another monooxygenase, propane monooxygenase (PrMO) is also expressed by Pseudonocardia dioxanivorans CB1190 during growth on 1,4-dioxane (Gedalanga et al. 2014). This enzyme’s role in 1,4-dioxane degradation is currently unknown, but it is not thought to be a reliable indicator for 1,4-dioxane-degrading activity in bacteria (Gedalanga et al. 2014).
Another well-characterized 1,4-dioxane-metabolizing strain, Mycobacterium dioxanitrophicus PH-06, has a lower Ks for 1,4-dioxane than Pseudonocardia dioxanivorans CB1190 and expresses similar levels of genes encoding two distinct monooxygenases when grown on 1,4-dioxane (He et al. 2017). The role of one of these enzymes, a copper-containing monooxygenase, in 1,4-dioxane degradation has not been established. The other enzyme, an iron-containing PrMO, can oxidize 1,4-dioxane, THF, and propane (Deng et al. 2018). The propane monooxygenase enzyme in Mycobacterium dioxanitrophicus PH-06 is distinctly different from the similarly named enzyme (PrMO) found in Pseudonocardia dioxanivorans CB1190 as well as many other bacteria with no known 1,4-dioxane-degrading activity. This is another example of where a misleading name for an enzyme can potentially cause confusion.
The pathway of 1,4-dioxane degradation by Pseudonocardia dioxanivorans CB1190 and Mycobacterium dioxanitrophicus PH-06 has been investigated, and the initial product of 1,4-dioxane oxidation in both microorganisms is 1,4-dioxane-2-ol [(Mahendra et al. 2007); (Kim et al. 2009)]. Studies have also detected 2-hydroxyethoxyacetic acid (2HEAA) as a 1,4-dioxane-derived metabolite ((Mahendra et al. 2007); (Vainberg et al. 2006)). In Pseudonocardia dioxanivorans CB1190, 2HEAA is further degraded to a series of two-carbon metabolites, including glycollate and glyoxylate (Mahendra et al. 2007). A potentially 2HEAA-independent pathway of 1,4-dioxane degradation involving 1,4-dioxane has also been described for several 1,4-dioxane-degrading bacteria [(Chen et al. 2016); (Huang et al. 2014)].
Some common metals (Cu>Cd>Ni>Zn) can inhibit the growth of 1,4-dioxane-metabolizing strains [(Pornwongthong et al. 2014); (Inoue et al. 2020)]. For example, copper was observed to have an inhibitory effect on 1,4-dioxane biodegradation by Pseudonocardia dioxanivorans CB1190 at a concentration of approximately 1 mg/L, whereas other metals tested—including cadmium, nickel, and zinc—did not have an effect until concentrations of 10 mg/L (Pornwongthong et al. 2014). Chlorinated co-contaminants that are frequently detected with 1,4-dioxane in ground water can also inhibit 1,4-dioxane degradation by Pseudonocardia dioxanivorans CB1190 (1,1-DCE > cis-1,2-DCE > TCE > 1,1,1-TCA) [(Zhang, Gedalanga, and Mahendra 2016); (Mahendra, Grostern, and Alvarez-Cohen 2013)]. The effects of these chlorinated co-contaminants are substantially attenuated when cells of Pseudonocardia dioxanivorans CB1190 are attached to surfaces rather than free living (planktonic) (Zhao et al. 2018).
Extensive laboratory studies have been conducted using strains Pseudonocardia dioxanivorans CB1190, Mycobacterium dioxanitrophicus PH-06, and others to understand the pathways and enzymes involved in bacterial 1,4-dioxane metabolism and some factors that may influence this process, such as the presence of heavy metals or specific chlorinated solvent co-contaminants [e.g., (Kim et al. 2009); (Mahendra and Alvarez-Cohen 2005); (Mahendra and Alvarez-Cohen 2005); (Pornwongthong et al. 2014); (Huang et al. 2014); (Zhang, Gedalanga, and Mahendra 2016)]. Organisms that carry out growth-linked biodegradation of 1,4-dioxane have also been used successfully in microcosm and column studies with site samples [(Kelley et al. 2001); (Da Silva et al. 2020); (Zhao et al. 2018)].
(Mora et al. 2020) conducted a recent field demonstration to assess the potential for Pseudonocardia dioxanivorans CB1190 to treat 1,4-dioxane in groundwater. In situ bioreactors (ISBRs) were deployed in monitoring wells for 6 months and consisted of packed beds of biological growth medium (Bio-Sep beads®) carrying Pseudonocardia dioxanivorans CB1190. Air sparging, nutrient addition, and in-well groundwater circulation were performed to maintain ideal conditions within the ISBRs for Pseudonocardia dioxanivorans CB1190 to biodegrade 1,4-dioxane. Multiple lines of evidence collected from inside the ISBRs over time indicated the occurrence of in situ biodegradation of 1,4-dioxane even in the presence of chlorinated solvents. The data collected from the study wells before and after ISBR deployment suggest that the ISBR approach can enhance the Pseudonocardia dioxanivorans CB1190 abundance and viability in the surrounding subsurface. Even with relatively low degradation rates, ISBRs can serve as a passive bioaugmentation strategy to maintain active functional microorganisms for long-term remediation.
6.5.2.4 In Situ Thermal Remediation
Due to the relatively high boiling point of 1,4-dioxane (101.5˚C) and low Henry’s Law constant at ambient temperatures, 1,4-dioxane is an inefficiently strippable compound. However, the boiling point of 1,4-dioxane decreases in the presence of water or moisture in soil. A positive azeotrope (see Section 3.1.1) occurs at 82% mass of 1,4-dioxane in water, resulting in a minimum boiling point of 87.82°C. At low part-per-million concentrations in water, the boiling point decreases to below that of water, making ISTR technologies potentially attractive to implement.
The Henry’s law constant for 1,4-dioxane at 25°C has been reported to be 0.0002 [dimensionless; (Vainberg et al. 2006)]. Prior testing has indicated that at the boiling point of water, 1,4-dioxane has a Henry’s law constant of 0.01 (dimensionless) resulting in a 50-fold increase from ambient temperature (Oberle, Crownover, and Kluger 2015). During boiling, the mass fraction of 1,4-dioxane in the steam will be approximately 11 times greater than the mass fraction of 1,4-dioxane in the water from which it was boiled (Schneider and Lynch 1943). As in situ steaming continues during the application of ISTR technologies, this drives the concentrations of 1,4-dioxane into solution exponentially toward zero, allowing for effective remediation of 1,4-dioxane using ISTR technologies.
ISTR is effective due to the substantial increase in Henry’s law constant at elevated temperatures and azeotropic behavior of 1,4-dioxane, causing a lower boiling point in the presence of water. Significant levels of 1,4-dioxane transition to the vapor phase at temperatures approaching the boiling point of water and concentrations of 1,4-dioxane in solution are driven exponentially toward zero. Once in the vapor phase, 1,4-dioxane can be treated using vapor-phase GAC.
The following subsections describe the ISTR technologies and important design parameters. Additionally, a combination of the technologies can be tailored based on site-specific characteristics to take advantage of the most desirable traits of each technology.
Electrical resistance heating
Electrical resistance heating (De Clercq et al.) is an ISTR technology that uses the resistance of soil to generate heat in the subsurface and reduce VOC concentrations in groundwater and soil. During ERH, the subsurface can be heated to the boiling point of water. Groundwater and soil moisture are converted to steam and, as a result, VOCs are removed via steam stripping and distillation.
ERH uses commonly available electricity and delivers it to the subsurface through electrodes. ERH electrodes can be installed vertically to any depth, at angles or horizontally underneath operating facilities and surface structures, and in the presence of buried utilities. The technology is equally effective in soil and most bedrock, and in the vadose and saturated zones. ERH passes an electrical current through the soil, rock, and groundwater that requires treatment. The principal current path is the thin layer of water immediately adjacent to the soil or rock grains. Relatively little current is carried by the water in the soil pores. The electrical current warms the subsurface and then boils a portion of the moisture into steam. This in situ steam generation occurs in all soil types, regardless of permeability, even in very low-permeability clay and rock. Sedimentary rock usually has significant primary porosity, and the rock grains generally have the thin film of water required for ERH.
The subsurface electrical energy evaporates the target contaminants and provides steam as a carrier gas to sweep VOCs to the vapor recovery wells. After condensing the steam and cooling the extracted air to ambient conditions, the vapor can be treated using conventional methods, such as GAC or thermal oxidizers. Important design parameters for an ERH design include treatment area, heating depth interval, primary and secondary contaminants of concern, VOC mass, electrical conductivity of soil, groundwater elevation, groundwater flow velocity, ERH electrode spacing, vapor recovery flow, utility power drop requirements, power and energy densities, and days of operation. The sweet spot for ERH is the volatilization and removal of VOCs, including 1,4-dioxane.
Thermal conduction heating
Thermal conduction heating (TCH) uses heaters, typically powered by electricity, to distribute heat throughout the subsurface. Through simple thermal conduction, heat propagates radially away from heater wells at a rate of approximately 1 inch per day. Heater wells are spaced systematically in a triangular pattern for optimal heat distribution. The heat fronts from each heater well will meet over the course of the remediation with thermal diffusivity limiting the rate of heat propagation in soil. The tighter the heater well spacing, the faster a site will heat up.
In contrast to other thermal technologies, TCH can achieve higher temperatures than the boiling point of water. Before temperatures above the boiling point of water can be achieved, all water must be boiled off; therefore, soil moisture has a profound impact on TCH energy requirements. While this higher temperature range can be advantageous for treatment of SVOCs and other contaminants with higher boiling points, it may not be necessary for 1,4-dioxane treatment. Important design parameters for a TCH design include treatment area, heating depth interval, heater well spacing, primary and secondary contaminants of concern, VOC mass, thermal diffusivity of soil, groundwater elevation, groundwater flow velocity, heater well spacing, vapor recovery flow, utility power drop requirements, power and energy densities, and days of operation. Because TCH can generate temperatures throughout the treatment volume beyond the boiling point of water, TCH can be more energy efficient than ERH for the removal of SVOCs.
Steam enhanced extraction
Steam enhanced extraction (SEE) is an ISTR technology used to heat higher permeability zones. During SEE, steam is injected into wells and migrates outward through permeable soils. Migrating steam and hot liquids, into which 1,4-dioxane may be entrained, are extracted from nearby multi-phase extraction wells. During SEE, steam is an amendment requiring delivery to the subsurface; therefore, heating is governed by the matrix’s hydraulic conductivity. Important design parameters for SEE design include treatment area, heating depth interval, ground surface cover, injection and extraction well spacing, nonaqueous phase liquid viscosity (if present), hydraulic conductivity of soil, groundwater elevation, groundwater flow velocity, and liquid treatment capacity. Because SEE is an injection and delivery ISTR method, it can be effective at treatment of 1,4-dioxane and displacement and treatment of nonaqueous phase liquids (NAPLs) within transmissive lithology.
6.5.3 Less Effective Technologies for Groundwater (In Situ)
Less effective technologies are just that, and typically include technologies with negligible or limited capability of 1,4-dioxane removal based on demonstration sites and/or theoretical considerations from 1,4-dioxane properties.
6.5.3.1 Anaerobic Bioremediation
In general, anaerobic biodegradation is a process in which microorganisms break down a contaminant under oxygen-deprived (aka highly reducing conditions). Under these circumstances, other terminal electron acceptors, such as nitrate, iron, sulfate, and others, are needed for bacteria respiration. In some cases, the contaminants themselves become a terminal electron acceptor. Anaerobic processes are usually much slower than their aerobic counterparts.
Biodegradation of 1,4-dioxane under anaerobic conditions remains an elusive approach since evidence found in the scientific literature is scarce. Given that many contaminated sites show anaerobic conditions with limited, or zero, dissolved oxygen concentrations in the groundwater, and that oxygen delivery is another step or barrier to overcome during aerobic biodegradation, anaerobic biodegradation of 1,4-dioxane is an important topic to be discussed.
Other ether compounds such as MTBE were believed to be recalcitrant under anaerobic conditions until it was demonstrated that they could be biodegraded in anaerobic sediments in petroleum-impacted groundwater (Finneran and Lovley 2001). 1,4-Dioxane, which has a closed ring structure and two ether bonds, may present a greater challenge. To date, only two peer-reviewed studies have provided evidence for anaerobic biodegradation of 1,4-dioxane. (Shen, Chen, and Pan 2008) showed that 25% of 1,4-dioxane was degraded in sludge samples after 40 days in an unamended treatment; treatments amended with iron oxide and humic acids showed up to 62% degradation, and treatments in which the iron was chelated with ethylenediaminetetraacetic acid (EDTA) and humic acids were present showed 90% degradation. A mineralization of 59% of 1,4-dioxane was found based on the carbon dioxide (CO2) measured directly in the headspace and as bicarbonate (HCO3–) and carbonate (CO32-) ions in the aqueous phase. However, no evidence of intermediate products nor any information about the microbial community responsible for this biodegradation was provided. (Ramalingam and Cupples 2020) tested multiple microcosms with various microbial inocula and electron acceptor amendments. Testing included use of CSIA and microbial community analysis to better understand the 1,4-dioxane concentration changes. Although 1,4-dioxane concentration decreases were observed, an anaerobic biodegradation pathway was not strongly confirmed.
Although other laboratory studies have attempted to replicate 1,4-dioxane anaerobic biodegradation, no sufficient evidence has been observed. Microcosms studies prepared with soil and groundwater under a variety of redox conditions using 14C-radio-labeled 1,4-dioxane showed no evidence for anaerobic biodegradation [(Arve 2015); (Barajas-Rodriguez 2016)].
In summary, efforts to demonstrate anaerobic biodegradation of 1,4-dioxane have not been successful since Shen, Chen, and Pan published their study in 2008. Therefore, anaerobic biodegradation of 1,4-dioxane is not an approach for remediation but remains an important, yet undemonstrated, topic.
6.5.3.2 Air Sparging/Soil Vapor Extraction
In situ air sparging is a commonly implemented remedial technology with relatively low costs compared to pump-and-treat approaches. Air sparging consists of injecting air into groundwater, mostly as bubbled air, to remove contaminants from the saturated zone via mass transfer (volatilization). Once out of the injection well, the air moves upward, creating a stripping zone. Volatile compounds will transfer from the groundwater into the air phase. The buoyant air bubbles are then directed toward a soil-venting system to capture the contaminants in the air phase. When air sparging is used to deliver oxygen for aerobic bioremediation by native or added microorganisms, the term becomes “biosparging” (Suthersan 1999).
An air sparging system comprises one or more subsurface locations in which air is injected into the saturated zone. The air is injected below or near a contaminant plume, rising back to the surface across the lithological formation, stripping the contaminants dissolved in the water, as well as nonaqueous phase organic compounds. Depending on the site conditions, the air may travel as bubbles or as continuous air channels. An SVE system is coupled with air sparging to channel the compounds in the gas phase above the water table. Mass transfer is one of the most dominant phenomena, and contaminants that are more volatile will be removed preferentially from the groundwater phase.
Because of 1,4-dioxane’s relatively low Henry’s law constant and high solubility in water, stripping 1,4-dioxane from groundwater via air sparging is challenging for in situ remediation. In this case, the relatively low volatility of 1,4-dioxane compared to other VOCs may be the most limiting factor for the implementability of this technique (Chiang et al. 2016). For these reasons, air sparging/SVE has a low effectiveness and poor implementability for 1,4-dioxane in situ remediation.
6.5.3.3 Zero-Valent Iron
ZVI is a chemical reductant that is commonly used for in situ chemical reduction (ISCR) treatment of chlorinated solvents. When treating PCE or TCE, by-products of ZVI reactions may include chloroacetylene, acetylene, and ethane. While cis-1,2-DCE and VC may be formed as well, these are not the primary degradation by-products as they are with reductive dechlorination. ZVI offers benefits for treatment of solvents, including rapid treatment (particularly for high initial concentrations of chlorinated solvents) and reaction pathways that include formation of unregulated by-products (e.g., chloroacetylene and acetylene). In addition, the process causes geochemically reducing conditions that are synergistic with reductive dechlorination treatment mechanisms that may be used in follow-on anaerobic bioremediation or MNA.
ZVI reactions do not degrade 1,4-dioxane. This has been found in laboratory batch studies [(Fan et al. 2017); (Zhong et al. 2015)] and in field-scale remediation projects (Chiang et al. 2016). This is also consistent with findings of research into reductive biological treatment mechanisms.
The discussion in this section relates to the treatment of 1,4-dioxane with ZVI used as a chemical reductant (i.e., the direct reactivity of ZVI with 1,4-dioxane). As discussed in Section 6.5.1.1.1, ZVI has also been used as an activator for persulfate in ISCO applications; refer to that section for guidance on ZVI as an activator for ISCO.