1.History of Use and Potential Sources
This section of the guidance document presents the history of manufacturing and intentional uses of 1,4-dioxane. It also discusses the unintentional existence of 1,4-dioxane in products due to chemical process by-products and potential related sources of contamination. This background information will aid users in deciding when characterization for 1,4-dioxane may be necessary.
1,4-Dioxane (also known as dioxane, p-dioxane, diethylene oxide, 1,4-diethylene dioxide, and glycol ethylene ether) is a synthetic organic compound currently and historically used in various industrial applications. Researchers first synthesized 1,4-dioxane (CAS Registry Number #123-91-1 (ACS 2019)) in 1863. In 1985, about 90% of the 1,4-dioxane produced was for use as a stabilizer for the chlorinated solvent 1,1,1-trichloroethane (1,1,1-TCA) (USEPA 1995c).
The U.S. Environmental Protection Agency (USEPA) has determined that 1,4-dioxane is likely to be carcinogenic to humans through all routes of exposure (USEPA 2013b). USEPA required national drinking water sampling for 1,4-dioxane under the Third Unregulated Contaminant Monitoring Rule (UCMR3), which was promulgated in 2012. (For additional information on the UCMR3, see Section 2.) 1,4-Dioxane has been detected in drinking water supplies, soil, and groundwater throughout the world (EC 2010). Therefore, understanding the historical uses of 1,4-dioxane, the sources of 1,4-dioxane, and the common 1,4-dioxane co-contaminants is necessary to identify sites where investigation may be required.
1.1 History of 1,4-Dioxane Production
Contamination of the environment by any chemical is generally linked to the chemical’s production and use. Release of chemicals from production facilities can result in high-mass, high-concentration sources, whereas releases by industrial consumers will be commensurate with the amount of product consumed.
Limited production of 1,4-dioxane began in 1929 (ACS 2019). Commercial-scale production of 1,4-dioxane began in 1951. Historically, 1,4-dioxane has primarily been used to stabilize 1,1,1-TCA; therefore, 1,4-dioxane’s production history is inextricably tied to the production and use of 1,1,1-TCA. Production of 1,4-dioxane spiked in the mid-1980s as companies phased out the use of trichloroethylene (TCE) in favor of 1,1,1-TCA (Figure 1-1). Demonstrating this growth, Surprenant (Surprenant 2012) provides two data points: in 1977, domestic 1,4-dioxane production was less than 2 million pounds annually, but by 1985, it had increased to approximately 25 million pounds annually. The production of 1,1,1-TCA also peaked in 1985 (Mohr et al. 2020). In 1985, approximately 90% of 1,4-dioxane produced was for the stabilization of 1,1,1-TCA (ECB 2002). 1,1,1-TCA use declined substantially post-1995 after it was identified as an ozone-depleting material pursuant to the 1987 Montreal Protocol (ATSDR 2012). By 2002, domestic annual production of 1,4-dioxane was estimated to be between 1 and 10 million pounds (ATSDR 2012).
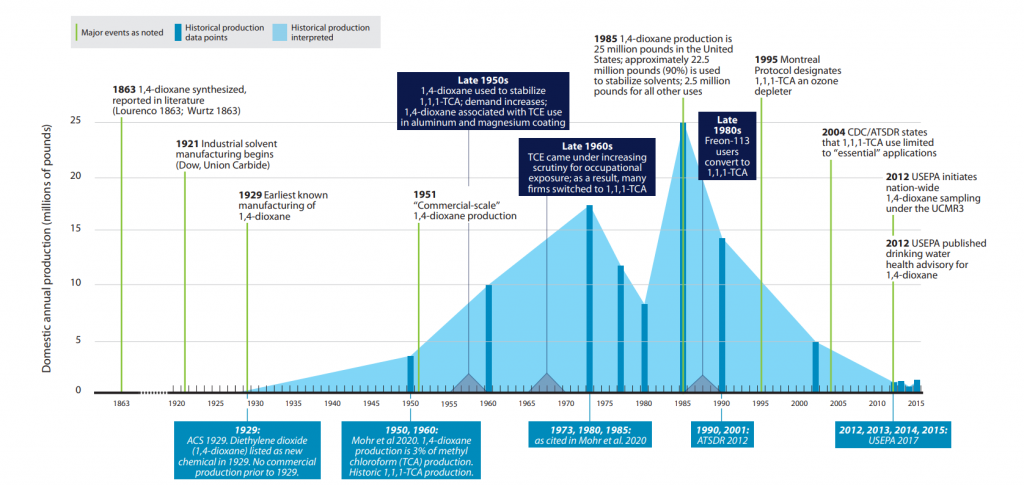
Figure 1-1. 1,4-Dioxane timeline and historic production.
Source: ITRC 1,4-Dioxane Team, 2020.
1,4-Dioxane is present as an impurity or unwanted by-product in some chemical processes. Companies produce significant quantities of 1,4-dioxane in polyethylene terephthalate (PET) and polyester plastic production (Hovenkamp and Munting 1970). Additionally, 1,4-dioxane is a by-product of some forms of ethoxylated surfactant production (Stepan 2006); (USEPA 2017d). 1,4-Dioxane may also be produced in the manufacture of detergents and cleaning compounds, especially those with components derived from ethoxylated surfactants. However, in some cases these processes have been modified to remove the 1,4-dioxane (ATSDR 2012). 1,4-Dioxane is also a common by-product of polyethylene glycol, an ethoxylated surfactant used in a wide variety of industries, including textiles, paper, lubricants, metal corrosion inhibitors, woodworking fluids, and oil and gas extraction hydraulic fracturing fluids. Polyethylene glycol products containing 1,4-dioxane in the range of 1 to 5 parts per million (ppm) have also been reported [(Dow 1989), as cited in (Mohr et al. 2020)].
1.2 Uses of 1,4-Dioxane
1.2.1 Solvent Stabilizers
1,4-Dioxane is most commonly associated with chlorinated solvents due to its use as a stabilizer in such solvents. Therefore, investigators should consider both the presence of chlorinated solvents (1,1,1-TCA, TCE, 1,1-dichloroethane [1,1-DCA], and 1,1-dichloroethene [1,1-DCE]) and the site history when evaluating the potential presence of 1,4-dioxane. Section 1.1 discusses how 1,4-dioxane’s history is linked to the production and use of 1,1,1-TCA and provides information on 1,4-dioxane’s association with TCE, 1,1-DCA, and 1,1-DCE.
Various industries and military operations have used chlorinated solvents for degreasing, for preparation of materials for chrome plating and other coatings, and for equipment repair and maintenance. Stabilizers are required to inhibit reactions between the solvent and the metals, which form acids as the solvent decomposes. Companies market chlorinated solvents as formulations containing potentially complex mixtures of stabilizing agents (Mohr et al. 2020). Figure 1-1 summarizes key milestones related to the historical production, use, and regulation of 1,4-dioxane as a stabilizing agent for chlorinated solvents. Details regarding regulations are contained in Section 2.
TCE and 1,1,1-TCA are two examples of chlorinated solvents that are formulated with solvent stabilizers to inhibit reactions between these solvents and metals such as aluminum (Mohr et al. 2020). The solvents typically are stabilized and then sold commercially. 1,1,1-TCA is an order of magnitude more reactive with aluminum than is TCE (Archer and Stevens 1977). Therefore 1,1,1-TCA requires a greater level of stabilization than does TCE. (Mohr et al. 2020) provide extensive documentation for the use of 1,4-dioxane as a stabilizing agent for 1,1,1-TCA.
Although it is relatively less reactive with aluminum and other metals than is 1,1,1-TCA, TCE has nevertheless been stabilized for vapor degreasing applications since at least the 1940s. The Department of Defense (DOD) specified “Trichloroethylene, Stabilized Degreasing” in a 1945 document (GSA 1945), although the specified stabilizer in that document was “aliphatic amines,” with no mention of 1,4-dioxane. However, other stabilizers such as 1,4-dioxane were acceptable for the DOD as long as the formulation met performance specifications. Other evidence of historically stabilized TCE formulations come from industry (NYSPPI 2017). Nonetheless, definitive documentation of 1,4-dioxane as a stabilizing agent for TCE is insufficient due to the lack of specificity in early patent literature describing TCE formulations (Morrison and Murphy 2015). Despite this lack of definitive documentation, given the increased use of 1,4-dioxane for solvent stabilization since the late 1950s and the existence of many different TCE manufacturers throughout the twentieth century, it is possible that some stabilized TCE contained 1,4-dioxane (Morrison and Murphy 2015).
While documentation of 1,4-dioxane stabilization of TCE may be scant, several studies have identified significant spatial correlation between 1,4-dioxane and TCE occurrence in groundwater across hundreds of contaminated sites [(Anderson, Anderson, and Bower 2012); (Adamson et al. 2014a); (Chiang et al. 2016); (Karges, Becker, and Püttmann 2018)]. Therefore, to determine if the stabilizer 1,4-dioxane is likely to be present at a site, you should consider the presence of TCE. In addition, TCE is more typically the driver for site characterization and remediation than is 1,1,1-TCA. This is generally because 1,1,1-TCA has both a significantly faster degradation rate (aka a lower half-life) (USEPA 1998e) and a significantly higher maximum contaminant level (MCL) (200 micrograms per liter [µg/L]) than TCE (5 µg/L). Therefore, 1,1,1-TCA often is not detected, and rarely exceeds its MCL, even at sites where it has historically been used. Because of 1,1,1-TCA’s faster degradation rate, its degradation products should be considered when evaluating the potential for 1,4-dioxane to be present at a site. 1,1,1-TCA degrades to 1,1-DCA via biological reductive dechlorination, and 1,1,1-TCA degrades to 1,1-DCE via abiotic degradation processes. Therefore, to determine if the stabilizer 1,4-dioxane is likely to be present at a site, the presence of 1,1-DCA and 1,1-DCE at any concentration should be considered.
The form of 1,1,1-TCA released in the environment (spilled product vs. waste solvent) can impact the potential to find 1,4-dioxane, as well as the extent of a potential 1,4-dioxane groundwater plume. While the proportion of 1,4-dioxane present in 1,1,1-TCA formulations was initially 2%–8% (IARC 1999), 1,1,1-TCA volatilizes and degrades more than 1,4-dioxane. As a result, 1,4-dioxane in spent 1,1,1-TCA is concentrated, approaching an estimated 20% by volume of the waste solvent (Mohr et al. 2020). The mass of 1,4-dioxane commingled with spent stabilized solvent at the time of a release is therefore potentially greater than the mass of 1,4-dioxane if neat solvent were released. See Section 3 for additional information about 1,4-dioxane fate and transport.
Finally, note that the presence of 1,4-dioxane in metal working and degreasing activities is not limited to the use of 1,1,1-TCA. 1,4-Dioxane was used in some cutting oils used in machining, at levels as high as 16.5% (Meike 1993, as cited in Mohr et al. 2020). These cutting oils could be carried in the waste TCE from degreasing operations, independent of any use of 1,1,1-TCA. Therefore, sites where TCE is detected, associated with these metal working processes, should also be considered for sampling of 1,4-dioxane.
1.2.2 Medical, Pharmaceutical, and Biotechnical
1,4-Dioxane has a variety of current and former uses in the medical, pharmaceutical, and biotechnical industries. It is primarily used as a solvent in the manufacture of pharmaceuticals, veterinary drugs, and natural health products; for research and development; and as an analytical reagent for laboratory use.
1,4-Dioxane is used in cellulose acetate production for kidney dialysis reverse osmosis membranes, which can be made from cellulose acetate yarn treated with 1,4-dioxane (Mohr et al. 2020). Scintillation cocktails, which use solvents, surfactants, and a scintillator to react with radio-labeled solutions, are used as tracers in the life sciences industry. Historically, scintillation cocktails contained as much as 80% 1,4-dioxane (Bray 1960). Modern scintillation cocktails include di-isopropylnaphthalene (DIPN) and phenyl-o-xylylethane (PXE) instead of 1,4-dioxane (Perkin-Elmer 2020). Medical and research laboratories also use 1,4-dioxane as a solvent for dehydrating tissue and preparing slides for microscopy [(Mohr et al. 2020); (Shearer and Hunsicker 1980)], preparing samples for analysis, and removing chrome-based staining (Winsor 2006). 1,4-Dioxane was used as a carrier for biocides, to analyze vegetable matter, to dehydrate biological samples prior to slide preparation, and to isolate DNA elements (Mohr et al. 2020). While some laboratories historically reclaimed 1,4-dioxane (Winsor 2006) after use, often the 1,4-dioxane-containing materials were discarded to wastewater treatment systems or landfills.
1,4-Dioxane is commonly used as a mold release agent for polyurethane manufacture (Mohr et al. 2020). Polyurethanes are widely used in medical devices such as feeding tubes and catheters. 1,4-Dioxane is also used in the pharmaceutical industry as a solvent, as well as in chemical synthesis and extraction of biological and natural products (USEPA 1997).
1.2.3 Rubber and Plastics
1,4-Dioxane is directly used in the rubber and plastics industry and can also be a by-product of certain sectors of the rubber and plastics industry. Manufacturers use 1,4-dioxane to make photosensitive resins and magnetic recording tapes (RSC 1992), acetate and cellulose triacetate (e.g., reverse osmosis and kidney dialysis membranes) [(Philippoff 2006), as cited in (Mohr et al. 2020)], and acetate cigarette filters (Matsumura, Shimamoto, and Shibata 1997). USEPA identified 1,4-dioxane as a waste produced as a manufacturing by-product from rubber and plastics industrial facilities (USEPA 2005a). USEPA Toxics Release Inventory (Adams, Scanlan, and Secrist) data show that releases of 1,4-dioxane from this industry declined from 11,261 pounds per year in 1993 to 614 pounds per year in 2001 (USEPA 2005b). The rubber and plastics industry transferred a reported 117,838 pounds of 1,4-dioxane to waste handling facilities, according to the 1995 TRI (USEPA 1995). The reported amount declined slightly to 112,206 pounds in 2001 (USEPA 2005b).
1.2.4 Inks, Paints, and Coatings
Industry has used 1,4-dioxane as a component of printing inks and paints since the 1950s (NYSPPI 2017), either as a solvent (to help ink/paint adhere to plastic) or in conjunction with 1,1,1-TCA (a solvent in some paints) (Mohr et al. 2020). As much as 5% of the 1,1,1-TCA production in the 1980s was for the inks and coatings industry, for use in cleaning printing equipment. An additional potential source for 1,4-dioxane in the printing industry is in the production of propylene glycol (Pundlik, Sitharaman, and Kaur 2001), which was used commonly in ink and paint formulation (Mohr et al. 2020). It was also used in the resins used to make paints and in paint and varnish strippers/removers, fabric dyes, and some felt-tip pens (Mohr et al. 2020). Some lacquer-based coatings contained as much as 60% 1,4-dioxane (AC 1987).
1.2.5 Adhesives
1,4-Dioxane was used as an additive in adhesives (Wilke, Jann, and Brödner 2004) for surface preparation prior to application of epoxy adhesives. It may also have been present as a contaminant in adhesives using 1,1,1-TCA as a solvent, which used to be a relatively common practice (HSDB 2019). 1,4-Dioxane concentrations in the 3%–5% range have been reported in wood glue, automotive trim adhesive, and contact cement. Indoor air detections of 1,4-dioxane are thought to be attributable to architectural coatings and adhesives used in the construction of office furniture (Mohr et al. 2020).
1.2.6 Automotive Fluids
Manufacturers included 1,4-dioxane in brake fluids, brake cleaning fluids, loosening fluids, and rust remover (Mohr et al. 2020). While some of the 1,4-dioxane was present because 1,1,1-TCA was used in these materials, Mohr (Mohr et al. 2020) found 1,4-dioxane concentrations higher than expected [(Valvoline 1991); (CRC 1987)] compared to 1,1,1-TCA concentrations. Therefore, 1,4-dioxane may have served other purposes in these applications. Automotive antifreeze formulations can contain 1,4-dioxane as an ingredient or impurity, with concentrations in the single-digit milligrams per liter (mg/L) range. The Agency for Toxic Substances and Disease Registry (ATSDR) noted 1,4-dioxane levels over 20 mg/L in radiator boilover (ATSDR 2012), suggesting creation of 1,4-dioxane in the glycol-based product with heat and pressure.
1.2.7 Aircraft Fluids
1,4-Dioxane was an additive or impurity in antifreeze and aircraft deicing fluids (UC 1989). Prior to 2000, most deicing fluids were glycol-based products, and the observation that 1,4-dioxane is a common manufacturing by-product in glycol production suggests widespread potential occurrence in areas impacted by deicing operations. 1,4-Dioxane levels have been reported in deicing fluids in the single-digit mg/L range (Pundlik, Sitharaman, and Kaur 2001).
1.2.8 Consumer Products
1,4-Dioxane is commonly present in raw materials used in the production of many consumer products, including cleaners, detergents, shampoos, and cosmetics. These include ethoxylated surfactants and other ingredients, including products containing propylene glycols [(Johnson 2001); (Pundlik, Sitharaman, and Kaur 2001)]. The process of acid- or base-catalyzed ethoxylation involved in the manufacture of many anionic, cationic, amphoteric, and nonionic surfactants generates 1,4-dioxane as a by-product (Mohr et al. 2020). The process of esterification of ethylene glycol in the manufacture of PET and polyester also generates 1,4-dioxane as a by-product (Mohr et al. 2020).
Testing in the early 1980s revealed 1,4-dioxane concentrations in consumer personal care products such as shampoos, bath gels, and lotions at levels up to 279 mg/L [(FDA 1980); (FDA 1981)]. Most products listing sodium laureth sulfate or ammonium laureth sulfate as ingredients have historically contained 1,4-dioxane in the 100–1,000 mg/L range (Mohr et al. 2020). Cosmetics are also commonly produced using ethoxylated surfactant agents and thus could contain 1,4-dioxane. In a study of cosmetic products in 1979, 1,4-dioxane was found at levels greater than 10 mg/L in 31 of 65 commercially available products (Fishbein 1981). Processing of consumer products has recently been modified to reduce 1,4-dioxane concentrations. Johnson & Johnson indicated that it has reformulated about 70% of its baby products to reduce 1,4-dioxane (Mohr et al. 2020).
Other industries may also use 1,4-dioxane as an extractant for processing food and consumer products. For example, products generated in the cannabis industry may contain 1,4-dioxane, as this industry uses a wide range of solvents to extract and process cannabidiol (CBD) oils (Mohr et al. 2020).
1.2.9 Pesticides
There is some evidence that past pesticide formulations contained 1,4-dioxane as an inert ingredient, including in the range of 20%–50% of the formulation, according to patent literature (Mohr et al. 2020). Additionally, pesticide patents have identified 1,4-dioxane present with solvents such as 1,1,1-TCA where 1,4-dioxane may have been a stabilizer for the 1,1,1-TCA (Chang 1999). USEPA listed 1,4-dioxane as an “inert [ingredient] of toxicological concern” in its Policy Statement on Inert Ingredients in Pesticide Products (USEPA 1987). However, according to the 2019 Annual Report on Risk Evaluations (USEPA 2019e), 1,4-dioxane was removed from the list of pesticide product inert ingredients in 1998 because it was no longer being used in pesticide products. It is also possible that trace amounts of 1,4-dioxane could be present in pesticides as part of the manufacture of ethoxylated surfactants, which are often found in pesticides (Mohr et al. 2020).
1.2.10 Other Uses
Officials have identified 1,4-dioxane in a wide variety of products. ATSDR found that 1,4-dioxane has been used in wood pulping, fumigants, laboratory applications, and polymerization (ATSDR 2012). Other uses include racing fuels (ProSystems 2002), fire retardant production (BCC 2002), medical device production (Wypych 2001), contraceptives (FDA 1997), herbicides (Diamond and Durkin 1997), and art restoration (Mohr et al. 2020). In addition, industry used 1,4-dioxane in cutting oils and coatings (Mohr et al. 2020) associated with metal parts fabrication and maintenance. Residuals from these products may have introduced 1,4-dioxane to TCE from use in the degreasing process. USEPA lists numerous other uses, which are not likely to be as widespread as the use as a chlorinated solvent stabilizer or uses associated with ethoxylated surfactant production (USEPA 2018e). These uses include the following:
- Solvent for ethyl cellulose, benzyl cellulose, waxes, and fats
- Spectroscopic and photometric measurements
- Wetting and dispersing agent in textile processing
- Degreasing agent
- Component of polishing compositions
- Production of plastic and rubber
1.3 Primary and Secondary Sources of Contamination
1.3.1 Wastewater Treatment
Wastewater treatment includes municipal sanitary waste treatment, industrial manufacturing waste treatment, and residential and commercial septic treatment. Industrial wastewater plants are considered primary sources because they are operating at production or manufacturing facilities generating the 1,4-dioxane, whereas sanitary wastewater treatment plants (WWTPs) represent a secondary source of 1,4-dioxane in that they receive rather than generate 1,4-dioxane. 1,4-Dioxane has been found widely in wastewater discharges to publicly owned treatment works (POTWs). Most standard forms of wastewater treatment are ineffective at removing 1,4-dioxane, so concentrations of 1,4-dioxane going into a POTW often remain unchanged in the effluent (Mohr et al. 2020). Low, persistent concentrations of 1,4-dioxane in wastewater effluent point to a widespread domestic source that could be due to contamination of consumer products—particularly personal care and cleaning products—with 1,4-dioxane (Simonich et al. 2013) or contamination of the local water supply [(Adamson, Piña, et al. 2017); (NYSPPI 2017)]. Studies have shown low parts per billion (ppb) concentrations of 1,4-dioxane in raw wastewater and treated effluent. 1,4-Dioxane concentrations in domestic wastewater effluents from 40 different WWTPs ranged from nondetect (<0.30 µg/L) to 3.30 µg/L with a mean of 1.11±0.60 µg/L (Simonich et al. 2013).
Industry sources, including plants producing PET plastic, pharmaceuticals, and soaps and detergents containing ethoxylated surfactants, can contribute to higher concentrations of 1,4-dioxane observed in some POTWs (Adamson, Piña, et al. 2017). Water is generated during the esterification process, and because 1,4-dioxane is miscible in water and is often not analyzed due to lack of regulatory requirements, 1,4-dioxane is often unwittingly processed with general wastewater. USEPA TRI data indicate 1,4-dioxane discharges from industrial facilities to surface water were common in the past (USEPA 2006). These discharges may still occur in facilities that use ethoxylation and esterification processes. Not all National Pollutant Discharge Elimination System (NPDES) and POTW permits require analysis for 1,4-dioxane; thus, discharges via NPDES outfalls and POTW effluent are a potential source of 1,4-dioxane in surface water (Stepien et al. 2014).
1.3.2 Landfill Waste and Leachate
Landfill waste materials and leachate, if released to the environment, are a potential secondary source of 1,4-dioxane. In other words, 1,4-dioxane is not produced at a landfill site but may instead be deposited at a landfill site through solid waste disposal. 1,4-Dioxane was found in 70% of landfills that receive incinerator wastes and 38% of landfills receiving noncombustible waste (Mohr et al. 2020). The higher levels in incinerator wastes may be from concentration of 1,4-dioxane from combustion of consumer products containing 1,4-dioxane in municipal solid waste (Fujiwara et al. 2008). In 2000, USEPA conducted a survey to review the chemistry of landfill leachate in the United States. The survey revealed the presence of 1,4-dioxane in leachate from construction and demolition (C&D) waste landfills (mean 1,4-dioxane concentration of 49 μg/L), municipal solid waste landfills (mean 1,4-dioxane concentration of 10.8 μg/L), and hazardous waste landfills (mean 1,4-dioxane concentration of 466 μg/L) (USEPA 2000).
1.3.3 Other Sources to Soil and Groundwater
Many historical disposal practices that were considered acceptable at the time have led to releases of 1,4-dioxane to the environment. These potential primary sources include septic drain fields, unlined lagoons and ponds, and burial of sludge in trenches. For example, 1,4-dioxane has been discovered in groundwater at concentrations of more than 250 mg/L at a San Jose, California, solvent recycling facility through inadvertent releases as well as improper disposal (ATSDR 2012). At Air Force Plant 44 in Tucson, Arizona, chlorinated solvent waste from degreasing operations was placed in an unlined lagoon between 1966 and 1970. This contributed to 1,4-dioxane concentrations as high as 600 µg/L in groundwater and a 6-mile long groundwater plume (ADEQ 2012). At the Pall-Gelman site in Ann Arbor, Michigan, widespread 1,4-dioxane impacts to groundwater were related to waste discharge to a series of unlined lagoons as well as spray irrigation of wastewater—all installed and operated with approval of the state Department of Natural Resources in the early to mid-1960s (Mohr et al. 2020). Septic leach field effluent, reclaimed water, and sewer line exfiltration inputs to groundwater sources may also serve as nonpoint sources of 1,4-dioxane contamination to groundwater and downgradient surface waters (Mohr et al. 2020).
Accidental releases, such as those from inadvertent spills or from leaking pipes, storage tanks, and drums, are also common primary sources of chlorinated solvent and associated 1,4-dioxane releases to the environment. A case study on the Seymour Recycling Corporation Superfund Site, a waste processing and storage facility in Indiana, documents USEPA enforcement actions related to 60,000 55-gallon drums and 98 bulk storage tanks containing various wastes, including substantial quantities of chlorinated solvent waste containing 1,4-dioxane (Mohr et al. 2020).
1.4 Presence in Environmental Media
1,4-Dioxane has been found in a wide range of media during investigations. The following sections briefly describe potentially impacted media and whether 1,4-dioxane is likely to be of environmental concern in those media. For information regarding physical and chemical properties of 1,4-dioxane, see Section 3.1.
1.4.1 Groundwater
1,4-Dioxane has been detected in groundwater in many regions of the world (EC 2010). Regional drinking water surveys have implicated 1,4-dioxane as a relatively widespread groundwater contaminant at levels of concern for human and environmental health [(Simazaki et al. 2006); (Adamson et al. 2017)]. Many 1,4-dioxane sites referenced in the literature are industrial sites with a history of chlorinated solvent use and disposal [(Abe 1999); (Anderson, Anderson, and Bower 2012); (Adamson et al. 2014a); (Karges, Becker, and Püttmann 2018)]. These industrial sites are often observed with large and dilute groundwater plumes [(Adamson et al. 2014a); (Chiang et al. 2016)] and thus have the potential to impact drinking water resources. Other examples of 1,4-dioxane groundwater contamination referenced in the literature include sites where 1,4-dioxane was specifically used or manufactured [see (Zenker, Borden, and Barlaz 2003) for a review], landfill leachate, car washes, wastewater effluent, and septic systems (Mohr et al. 2020). Refer to Section 1.3 for information on sources of contamination at sites. A U.S. Geological Survey (USGS) study looked at groundwater near a former waste-oil refinery in Indiana between 1997 and 2000 (ATSDR 2012). The site operated over 50 years until 1987 and included typical refinery facilities (e.g., storage tanks, cracking towers, and waste-oil storage lagoons). 1,4-Dioxane groundwater concentrations ranged from 3–31,000 μg/L (ATSDR 2012).
1.4.2 Surface Water
Surface water contamination from 1,4-dioxane primarily originates from diverse industrial and municipal sources, including industrial and sanitary waste treatment discharges, surface runoff from impacted sites, and groundwater discharge to surface water [(Han, So, and Kim 2000); (Nahar and Zhang 2011); (Mohr et al. 2020)]. Newell et al. (Newell et al. 2020) recently published a comparison to other common contaminants that indicated that 1,4-dioxane was detected in 12% of public water supply samples versus 18% for chlorinated volatile organic compounds (CVOCs), 1.3% for benzene, and 1% for per- and polyfluoroalkyl substances (PFAS). They estimated that 1,4-dioxane is likely to be present at 23,000 sites, versus 53,000 for CVOCs, 560,000 for benzene, and 42,560 for PFAS (Newell et al. 2020).
Sampling under the UCMR3 indicated that approximately 10% of surface water sources had detections of 1,4-dioxane, with a median concentration of 0.15 μg/L, suggesting relatively widespread impacts (Adamson, Piña, et al. 2017). UCMR3 data have also identified 1,4-dioxane in treated drinking water from surface water sources (USEPA 2017f). TRI data indicate 56,996 pounds discharged to surface water in 2006, representing 31% of the estimated total on-site environmental releases from facilities required to report to the TRI (ATSDR 2012). The USGS study mentioned in Section 1.4.1 also sampled surface water outside the Indiana refinery. 1,4-Dioxane levels ranged from 8–140 μg/L in the surface water collected from the network of ditches surrounding the site (ATSDR 2012). In the Cape Fear River Basin in coastal North Carolina, 1,4-dioxane levels in four surface water monitoring locations exhibited maximum and mean values ranging from 171–1,030 μg/L and 43– 351 μg/L, respectively [(NCDWR 2016), as cited in (Mohr et al. 2020)].
1.4.3 Soil
Because of its miscibility in water, low vapor pressure, and minimal sorption to organic carbon, 1,4-dioxane is rarely found at levels of concerns in soil (see Section 3.1.2 for more information on factors affecting the transport of 1,4-dioxane in the subsurface). Laboratory column and field studies determined groundwater migration retardation factors between 1.0 and 1.6 (Mohr et al. 2020), suggesting that 1,4-dioxane would move through a soil column close to the rate of water (such as infiltration from precipitation or groundwater). These estimated low retardation factors suggest minimal 1,4-dioxane retention in soil. TRI data indicate 596 pounds were discharged to soils in 2007, representing <1% of the estimated total on-site environmental releases from facilities required to report to the TRI (ATSDR 2012). A literature search yielded limited examples of bulk soil concentration data for 1,4-dioxane. At McClellan Air Force Base in California, soil sampling at a former solvent waste disposal landfill exhibited concentrations as high as 30 mg/kg (Hinchee 2017). Soil from the perimeter of a polyester wastewater treatment lagoon near Salisbury, North Carolina, contained 1,4-dioxane at 21.0 mg/kg (Aitchison et al. 2000).
1.4.4 Air
1,4-Dioxane may be released to air during its production, its use (ATSDR 2012), and in the processing of other chemicals (such as pharmaceuticals or pesticides). In 1984, the concentration of 1,4-dioxane ranged from 0.1–0.4 μg/m3 in ambient air sampled in the United States. This published study contains no information on the source of the 1,4-dioxane detected or the locations of the air sampling (EC 2010). In air, 1,4-dioxane remains as a vapor but readily degrades through reactions with photochemically produced hydroxyl radicals (USEPA 2014b) (see Section 3.1.6 for additional information on photodegradation).
Because the use of 1,4-dioxane has declined in recent years, current levels of 1,4-dioxane in ambient air are likely to be lower than the levels reported in the 1980s or earlier. However, recent national surveys are unavailable. USEPA TRI data indicate that 125,341 pounds was discharged to the atmosphere in 2007, representing 69% of the estimated total on-site environmental releases from facilities required to report to the TRI (ATSDR 2012). 1,4-Dioxane has been detected in ambient air monitoring samples in a few isolated studies. A compilation of ambient air sampling at 45 locations in 12 cities in the late 1970s to early 1980s indicated 1,4-dioxane levels up to 30 µg/m3 with a mean concentration of 0.44 µg/m3 [(USEPA 1993), as cited in (Mohr et al. 2020)]. In 1981, a study of ambient air in several industrial areas in New Jersey exhibited a 1,4-dioxane detection rate of 51% and mean values ranging from 0.04–0.07 µg/m3 (Mohr et al. 2020).